- DNA methylation
-
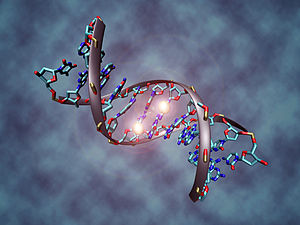 Illustration of a DNA molecule that is methylated at the two center cytosines. DNA methylation plays an important role for epigenetic gene regulation in development and disease.
Illustration of a DNA molecule that is methylated at the two center cytosines. DNA methylation plays an important role for epigenetic gene regulation in development and disease.
DNA methylation is a biochemical process that is important for normal development in higher organisms. It involves the addition of a methyl group to the 5 position of the cytosine pyrimidine ring or the number 6 nitrogen of the adenine purine ring (cytosine and adenine are two of the four bases of DNA). This modification can be inherited through cell division.
DNA methylation is a crucial part of normal organismal development and cellular differentiation in higher organisms. DNA methylation stably alters the gene expression pattern in cells such that cells can "remember where they have been" or decrease gene expression; for example, cells programmed to be pancreatic islets during embryonic development remain pancreatic islets throughout the life of the organism without continuing signals telling them that they need to remain islets. DNA methylation is typically removed during zygote formation and re-established through successive cell divisions during development. However, the latest research shows that hydroxylation of methyl group occurs rather than complete removal of methyl groups in zygote.[1] Some methylation modifications that regulate gene expression are inheritable and are referred to as epigenetic regulation.
In addition, DNA methylation suppresses the expression of viral genes and other deleterious elements that have been incorporated into the genome of the host over time. DNA methylation also forms the basis of chromatin structure, which enables cells to form the myriad characteristics necessary for multicellular life from a single immutable sequence of DNA. DNA methylation also plays a crucial role in the development of nearly all types of cancer.[2]
DNA methylation at the 5 position of cytosine has the specific effect of reducing gene expression and has been found in every vertebrate examined. In adult somatic tissues, DNA methylation typically occurs in a CpG dinucleotide context; non-CpG methylation is prevalent in embryonic stem cells.[3][4][5]
Contents
In mammals
DNA methylation is essential for normal development and is associated with a number of key processes including genomic imprinting, X-chromosome inactivation, suppression of repetitive elements, and carcinogenesis.
Between 60% and 90% of all CpGs are methylated in mammals.[6][7] Methylated C residues spontaneously deaminate to form T residues over evolutionary time; hence CpG dinucleotides steadily mutate to TpG dinucleotides, which is evidenced by the under-representation of CpG dinucleotides in the human genome (they occur at only 21% of the expected frequency).[8] (On the other hand, spontaneous deamination of unmethylated C residues gives rise to U residues, a mutation that is quickly recognized and repaired by the cell.)
Unmethylated CpGs are often grouped in clusters called CpG islands, which are present in the 5' regulatory regions of many genes. In many disease processes, such as cancer, gene promoter CpG islands acquire abnormal hypermethylation, which results in transcriptional silencing that can be inherited by daughter cells following cell division. Alterations of DNA methylation have been recognized as an important component of cancer development. Hypomethylation, in general, arises earlier and is linked to chromosomal instability and loss of imprinting, whereas hypermethylation is associated with promoters and can arise secondary to gene (oncogene suppressor) silencing, but might be a target for epigenetic therapy.[9]
DNA methylation may affect the transcription of genes in two ways. First, the methylation of DNA itself may physically impede the binding of transcriptional proteins to the gene, and second, and likely more important, methylated DNA may be bound by proteins known as methyl-CpG-binding domain proteins (MBDs). MBD proteins then recruit additional proteins to the locus, such as histone deacetylases and other chromatin remodeling proteins that can modify histones, thereby forming compact, inactive chromatin, termed heterochromatin. This link between DNA methylation and chromatin structure is very important. In particular, loss of methyl-CpG-binding protein 2 (MeCP2) has been implicated in Rett syndrome; and methyl-CpG-binding domain protein 2 (MBD2) mediates the transcriptional silencing of hypermethylated genes in cancer.
Research has suggested that long-term memory storage in humans may be regulated by DNA methylation.[10][11]
In cancer
DNA methylation is an important regulator of gene transcription and a large body of evidence has demonstrated that aberrant DNA methylation is associated with unscheduled gene silencing, and the genes with high levels of 5-methylcytosine in their promoter region are transcriptionally silent. DNA methylation is essential during embryonic development, and in somatic cells, patterns of DNA methylation are generally transmitted to daughter cells with a high fidelity. Aberrant DNA methylation patterns have been associated with a large number of human malignancies and found in two distinct forms: hypermethylation and hypomethylation compared to normal tissue. Hypermethylation is one of the major epigenetic modifications that repress transcription via promoter region of tumour suppressor genes. Hypermethylation typically occurs at CpG islands in the promoter region and is associated with gene inactivation. Global hypomethylation has also been implicated in the development and progression of cancer through different mechanisms.[12]
DNA methyltransferases
In mammalian cells, DNA methylation occurs mainly at the C5 position of CpG dinucleotides and is carried out by two general classes of enzymatic activities – maintenance methylation and de novo methylation.[citation needed]
Maintenance methylation activity is necessary to preserve DNA methylation after every cellular DNA replication cycle. Without the DNA methyltransferase (DNMT), the replication machinery itself would produce daughter strands that are unmethylated and, over time, would lead to passive demethylation. DNMT1 is the proposed maintenance methyltransferase that is responsible for copying DNA methylation patterns to the daughter strands during DNA replication. Mouse models with both copies of DNMT1 deleted are embryonic lethal at approximately day 9, due to the requirement of DNMT1 activity for development in mammalian cells.
It is thought that DNMT3a and DNMT3b are the de novo methyltransferases that set up DNA methylation patterns early in development. DNMT3L is a protein that is homologous to the other DNMT3s but has no catalytic activity. Instead, DNMT3L assists the de novo methyltransferases by increasing their ability to bind to DNA and stimulating their activity. Finally, DNMT2 (TRDMT1) has been identified as a DNA methyltransferase homolog, containing all 10 sequence motifs common to all DNA methyltransferases; however, DNMT2 (TRDMT1) does not methylate DNA but instead methylates cytosine-38 in the anticodon loop of aspartic acid transfer RNA.[13]
Since many tumor suppressor genes are silenced by DNA methylation during carcinogenesis, there have been attempts to re-express these genes by inhibiting the DNMTs. 5-Aza-2'-deoxycytidine (decitabine) is a nucleoside analog that inhibits DNMTs by trapping them in a covalent complex on DNA by preventing the β-elimination step of catalysis, thus resulting in the enzymes' degradation. However, for decitabine to be active, it must be incorporated into the genome of the cell, which can cause mutations in the daughter cells if the cell does not die. In addition, decitabine is toxic to the bone marrow, which limits the size of its therapeutic window. These pitfalls have led to the development of antisense RNA therapies that target the DNMTs by degrading their mRNAs and preventing their translation. However, it is currently unclear whether targeting DNMT1 alone is sufficient to reactivate tumor suppressor genes silenced by DNA methylation.
In plants
Significant progress has been made in understanding DNA methylation in the model plant Arabidopsis thaliana. DNA methylation in plants differs from that of mammals: while DNA methylation in mammals mainly occurs on the cytosine nucleotide in a CpG site, in plants the cytosine can be methylated at CpG, CpHpG, and CpHpH sites, where H represents any nucleotide but guanine.
The principal Arabidopsis DNA methyltransferase enzymes, which transfer and covalently attach methyl groups onto DNA, are DRM2, MET1, and CMT3. Both the DRM2 and MET1 proteins share significant homology to the mammalian methyltransferases DNMT3 and DNMT1, respectively, whereas the CMT3 protein is unique to the plant kingdom. There are currently two classes of DNA methyltransferases: 1) the de novo class, or enzymes that create new methylation marks on the DNA; and 2) a maintenance class that recognizes the methylation marks on the parental strand of DNA and transfers new methylation to the daughters strands after DNA replication. DRM2 is the only enzyme that has been implicated as a de novo DNA methyltransferase. DRM2 has also been shown, along with MET1 and CMT3 to be involved in maintaining methylation marks through DNA replication.[14] Other DNA methyltransferases are expressed in plants but have no known function (see the Chromatin Database).
It is not clear how the cell determines the locations of de novo DNA methylation, but evidence suggests that, for many (though not all) locations, RNA-directed DNA methylation (RdDM) is involved. In RdDM, specific RNA transcripts are produced from a genomic DNA template, and this RNA forms secondary structures called double-stranded RNA molecules.[15] The double-stranded RNAs, through either the small interfering RNA (siRNA) or microRNA (miRNA) pathways direct de-novo DNA methylation of the original genomic location that produced the RNA.[15] This sort of mechanism is thought to be important in cellular defense against RNA viruses and/or transposons, both of which often form a double-stranded RNA that can be mutagenic to the host genome. By methylating their genomic locations, through an as yet poorly-understood mechanism, they are shut off and are no longer active in the cell, protecting the genome from their mutagenic effect.
In fungi
It can be seen that many fungi have low levels (0.1 to 0.5%) of cytosine methylation, whereas other fungi have as much as 5% of the genome methylated.[16]
This value seems to vary both among species and among isolates of the same species.[17] There is also evidence that DNA methylation may be involved in state-specific control of gene expression in fungi.[citation needed]
Although brewers' yeast (Saccharomyces) and fission yeast (Schizosaccharomyces) have very little DNA methylation, the model filamentous fungus Neurospora crassa has a well-characterized methylation system.[18] Several genes control methylation in Neurospora and mutation of the DNA methyl transferase, dim-2, eliminates all DNA methylation but does not affect growth or sexual reproduction. While the Neurospora genome has very little repeated DNA, half of the methylation occurs in repeated DNA including transposon relics and centromeric DNA. The ability to evaluate other important phenomena in a DNA methylase-deficient genetic background makes Neurospora an important system in which to study DNA methylation.
In bacteria
Adenine or cytosine methylation is part of the restriction modification system of many bacteria, in which specific DNA sequences are methylated periodically throughout the genome. A methylase is the enzyme that recognizes a specific sequence and methylates one of the bases in or near that sequence. Foreign DNAs (which are not methylated in this manner) that are introduced into the cell are degraded by sequence-specific restriction enzymes and cleaved. Bacterial genomic DNA is not recognized by these restriction enzymes. The methylation of native DNA acts as a sort of primitive immune system, allowing the bacteria to protect themselves from infection by bacteriophage.
E. coli DNA adenine methyltransferase (Dam) is an enzyme of ~32 kDa that does not belong to a restriction/modification system. The target recognition sequence for E. coli Dam is GATC, as the methylation occurs at the N6 position of the adenine in this sequence (G meATC). The three base pairs flanking each side of this site also influence DNA–Dam binding. Dam plays several key roles in bacterial processes, including mismatch repair, the timing of DNA replication, and gene expression. As a result of DNA replication, the status of GATC sites in the E. coli genome changes from fully methylated to hemimethylated. This is because adenine introduced into the new DNA strand is unmethylated. Re-methylation occurs within two to four seconds, during which time replication errors in the new strand are repaired. Methylation, or its absence, is the marker that allows the repair apparatus of the cell to differentiate between the template and nascent strands. It has been shown that altering Dam activity in bacteria results in increased spontaneous mutation rate. Bacterial viability is compromised in dam mutants that also lack certain other DNA repair enzymes, providing further evidence for the role of Dam in DNA repair.
One region of the DNA that keeps its hemimethylated status for longer is the origin of replication, which has an abundance of GATC sites. This is central to the bacterial mechanism for timing DNA replication. SeqA binds to the origin of replication, sequestering it and thus preventing methylation. Because hemimethylated origins of replication are inactive, this mechanism limits DNA replication to once per cell cycle.
Expression of certain genes, for example those coding for pilus expression in E. coli, is regulated by the methylation of GATC sites in the promoter region of the gene operon. The cells' environmental conditions just after DNA replication determine whether Dam is blocked from methylating a region proximal to or distal from the promoter region. Once the pattern of methylation has been created, the pilus gene transcription is locked in the on or off position until the DNA is again replicated. In E. coli, these pilus operons have important roles in virulence in urinary tract infections. It has been proposed[by whom?] that inhibitors of Dam may function as antibiotics.
On the other hand, DNA cytosine methylase targets CCAGG and CCTGG sites to methylate cytosine at the C5 position (C meC(A/T)GG). The other methylase enzyme, EcoKI, causes mehtylation of adenine in the sequences AAC(N6A)GTGC and GCAC(N6A)GTT.
Most strains used by molecular biologists are derivatives of K-12, and possess both Dam and Dcm, but there are commercially available strains that possess dam-/dcm- activity. In fact, it is possible to unmethylate the DNA extracted from dam+/dcm+ strains by transforming into dam-/dcm- strains. This would help digest sequences that are not being recognized by methylation-sensitive restriction enzymes.[19][20]
Detection
DNA methylation can be detected by the following assays currently used in scientific research:
- Methylation-Specific PCR (MSP), which is based on a chemical reaction of sodium bisulfite with DNA that converts unmethylated cytosines of CpG dinucleotides to uracil or UpG, followed by traditional PCR. However, methylated cytosines will not be converted in this process, and primers are designed to overlap the CpG site of interest, which allows one to determine methylation status as methylated or unmethylated.
- Whole genome bisulfite sequencing, also known as BS-Seq, which is a high-throughput genome-wide analysis of DNA methylation. It is based on aforementioned sodium bisulfite conversion of genomic DNA, which is then sequencing on a Next-generation sequencing platform. The sequences obtained are then re-aligned to the reference genome to determine methylation states of CpG dinucleotides based on mismatches resulting from the conversion of unmethylated cytosines into uracil.
- The HELP assay, which is based on restriction enzymes' differential ability to recognize and cleave methylated and unmethylated CpG DNA sites.
- ChIP-on-chip assays, which is based on the ability of commercially prepared antibodies to bind to DNA methylation-associated proteins like MeCP2.
- Restriction landmark genomic scanning, a complicated and now rarely-used assay based upon restriction enzymes' differential recognition of methylated and unmethylated CpG sites; the assay is similar in concept to the HELP assay.
- Methylated DNA immunoprecipitation (MeDIP), analogous to chromatin immunoprecipitation, immunoprecipitation is used to isolate methylated DNA fragments for input into DNA detection methods such as DNA microarrays (MeDIP-chip) or DNA sequencing (MeDIP-seq).
- Pyrosequencing of bisulfite treated DNA. This is sequencing of an amplicon made by a normal forward primer but a biatenylated reverse primer to PCR the gene of choice. The Pyrosequencer then analyses the sample by denaturing the DNA and adding one nucleotide at a time to the mix according to a sequence given by the user. If there is a mis-match, it is recorded and the percentage of DNA for which the mis-match is present is noted. This gives the user a percentage methylation per CpG island.
- Molecular break light assay for DNA adenine methyltransferase activity – an assay that relies on the specificity of the restriction enzyme DpnI for fully methylated (adenine methylation) GATC sites in an oligonucleotide labeled with a fluorophore and quencher. The adenine methyltransferase methylates the oligonucleotide making it a substrate for DpnI. Cutting of the oligonucleotide by DpnI gives rise to a fluorescence increase.[21][22]
- Methyl Sensitive Southern Blotting is similar to the HELP assay, although uses Southern blotting techniques to probe gene-specific differences in methylation using restriction digests. This technique is used to evaluate local methylation near the binding site for the probe.
See also
- Demethylating agent
- MethDB DNA Methylation database
- Reprogramming
- Epigenetics, of which DNA methylation is a significant contributor
- Genomic imprinting, an inherited repression of an allele, relying on DNA methylation
- 5-Hydroxymethylcytosine
References
- ^ Iqbal, K.; Jin, S.-G.; Pfeifer, G. P.; Szabo, P. E. (2011). "Reprogramming of the paternal genome upon fertilization involves genome-wide oxidation of 5-methylcytosine". Proceedings of the National Academy of Sciences 108 (9): 3642–3647. doi:10.1073/pnas.1014033108. PMC 3048122. PMID 21321204. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3048122.
- ^ Jaenisch, R.; Bird, A. (2003). "Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals". Nature genetics 33 Suppl (3s): 245–254. doi:10.1038/ng1089. PMID 12610534.
- ^ Dodge JE, Ramsahoye BH, Wo ZG, Okano M, Li E (2002). "De novo methylation of MMLV provirus in embryonic stem cells: CpG versus non-CpG methylation". Gene 289 (1–2): 41–48. doi:10.1016/S0378-1119(02)00469-9.
- ^ Haines TR, Rodenhiser DI, Ainsworth PJ (2001). "Allele-Specific Non-CpG Methylation of the Nf1 Gene during Early Mouse Development". Developmental Biology 240 (2): 585–598. doi:10.1006/dbio.2001.0504. PMID 11784085.
- ^ Lister R, Pelizzola M, Dowen RH, et al. (October 2009). "Human DNA methylomes at base resolution show widespread epigenomic differences". Nature 462 (7271): 315–22. doi:10.1038/nature08514. PMC 2857523. PMID 19829295. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2857523.
- ^ Ehrlich M, Gama Sosa MA, Huang L-H., Midgett RM, Kuo KC, McCune RA, Gehrke C (April 1982). "Amount and distribution of 5-methylcytosine in human DNA from different types of tissues or cells". Nucleic Acids Research 10 (8): 2709–2721. doi:10.1093/nar/10.8.2709. PMC 320645. PMID 7079182. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=320645.
- ^ Tucker KL (June 2001). "Methylated cytosine and the brain: a new base for neuroscience". Neuron 30 (3): 649–652. doi:10.1016/S0896-6273(01)00325-7. PMID 11430798.
- ^ International Human Genome Sequencing Consortium, et al. (February 2001). "Initial sequencing and analysis of the human genome". Nature 409 (6822): 860–921. doi:10.1038/35057062. PMID 11237011.
- ^ Daura-Oller E, Cabre M, Montero MA, Paternain JL, Romeu A (2009). "Specific gene hypomethylation and cancer: New insights into coding region feature trends". Bioinformation 3 (8): 340–343. PMC 2720671. PMID 19707296. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2720671.
- ^ Miller C, Sweatt J (2007-03-15). "Covalent modification of DNA regulates memory formation". Neuron 53 (6): 857–869. doi:10.1016/j.neuron.2007.02.022. PMID 17359920.
- ^ Powell, Devin (2008-12-02). "Memories may be stored on your DNA". New Scientist. http://www.newscientist.com/article/mg20026845.000-memories-may-be-stored-on-your-dna.html. Retrieved 2008-12-02.
- ^ Craig, JM; Wong, NC (editor) (2011). Epigenetics: A Reference Manual. Caister Academic Press. ISBN 978-1-904455-88-2.
- ^ Goll MG, Kirpekar F, Maggert KA, Yoder JA, Hsieh CL, Zhang X, Golic KG, Jacobsen SE, Bestor TH (January 2006). "Methylation of tRNAAsp by the DNA methyltransferase homolog Dnmt2". Science 311 (5759): 395–398. doi:10.1126/science.1120976. PMID 16424344.
- ^ Cao X and Jacobsen SE (December 2002). "Locus-specific control of asymmetric and CpNpG methylation by the DRM and CMT3 methyltransferase genes". PNAS 99 (Suppl 4): 16491–16498. doi:10.1073/pnas.162371599. PMC 139913. PMID 12151602. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=139913.
- ^ a b Aufsatz W, Mette MF, van der Winden J, Matzke AJM, Matzke M (2002). "RNA-directed DNA methylation in Arabidopsis". PNAS 99 (90004): 16499–16506. doi:10.1073/pnas.162371499. PMC 139914. PMID 12169664. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=139914.
- ^ Antequera F, Tamame M, Villanueva JR, Santos T (July 1984). "DNA methylation in the fungi". J. Biol. Chem. 259 (13): 8033–8036. PMID 6330093.
- ^ Binz T, D'Mello N, Horgen PA (1998). "A comparison of DNA methylation levels in selected isolates of higher fungi". Mycologia (Mycological Society of America) 90 (5): 785–790. doi:10.2307/3761319. JSTOR 3761319.
- ^ Selker EU, Tountas NA, Cross SH, Margolin BS, Murphy JG, Bird AP, Freitag M (2003). "The methylated component of the Neurospora crassa genome". Nature 422 (6934): 893–897. doi:10.1038/nature01564. PMID 12712205.
- ^ Palmer BR and Marinus MG (1994). "The dam and dcm strains of Escherichia coli—a review". Gene 143 (1): 1–12. doi:10.1016/0378-1119(94)90597-5. PMID 8200522.
- ^ "Making unmethylated (dam-/dcm-) DNA". http://www.neb.com/nebecomm/tech_reference/restriction_enzymes/making_unmethylated_dna.asp.
- ^ Wood RJ, Maynard-Smith MD, Robinson VL, Oyston PC, Titball RW, Roach PL (2007). Fugmann, Sebastian. ed. "Kinetic analysis of Yersinia pestis DNA adenine methyltransferase activity using a hemimethylated molecular break light oligonucleotide". PLoS ONE 2 (8): e801. doi:10.1371/journal.pone.0000801. PMC 1949145. PMID 17726531. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1949145.
- ^ Li J, Yan H, Wang K, Tan W, Zhou X (February 2007). "Hairpin fluorescence DNA probe for real-time monitoring of DNA methylation". Anal. Chem. 79 (3): 1050–1056. doi:10.1021/ac061694i. PMID 17263334.
Further reading
- Law J, Jacobsen SE (2010). "Establishing, maintaining and modifying DNA methylation patterns in plants and animals". Nat. Rev. Genet. 11 (3): 204–220. doi:10.1038/nrg2719. PMC 3034103. PMID 20142834. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3034103.
- Straussman R, Nejman D, Roberts D, et al. (2009). "Developmental programming of CpG island methylation profiles in the human genome". Nat. Struct. Mol. Biol. 16 (5): 564–571. doi:10.1038/nsmb.1594. PMID 19377480.
- Patra SK (2008). "Ras regulation of DNA-methylation and cancer". Exp Cell Res 314 (6): 1193–1201. doi:10.1016/j.yexcr.2008.01.012. PMID 18282569.
- Patra SK, Patra A, Ghosh TC, et al. (2008). "Demethylation of (cytosine-5-C-methyl) DNA and regulation of transcription in the epigenetic pathways of cancer development". Cancer Metast. Rev. 27 (2): 315–334. doi:10.1007/s10555-008-9118-y. PMID 18246412.
External links
Gene expression Introduction to genetics Transcription (Transcription factors, RNA Polymerase,promoter) Prokaryotic / Archaeal / Eukaryotic
post-transcriptional modification (hnRNA,5' capping,Splicing,Polyadenylation)Translation (Ribosome,tRNA) Prokaryotic / Archaeal / Eukaryotic
post-translational modification (functional groups, peptides, structural changes)Gene regulation Transcriptional regulation prokaryoticeukaryoticHistone-modifying enzymes (histone/nucleosome): Histone methylation/Histone methyltransferase (EZH2) · Histone demethylase · Histone acetylation and deacetylation (Histone deacetylase HDAC1 · Histone acetyltransferase)
DNA methylation (DNA methyltransferase)
Chromatin remodeling: CHD7bothPromotion Promoter (Pribnow box, TATA box, BRE, CAAT box, Response element) · Enhancer (E-box, Response element) · Insulator · SilencerInitiation (prokaryotic,
eukaryotic)Transcription start siteElongation Termination
(prokaryotic,
eukaryotic)see also disorders of transcription and post transcriptional modification
B bsyn: dna (repl, cycl, reco, repr) · tscr (fact, tcrg, nucl, rnat, rept, ptts) · tltn (risu, pttl, nexn) · dnab, rnab/runp · stru (domn, 1°, 2°, 3°, 4°)Categories:
Wikimedia Foundation. 2010.
