- Carcinogenesis
-
For the scientific journal, see Carcinogenesis (journal).
Carcinogenesis or oncogenesis is literally the creation of cancer. It is a process by which normal cells are transformed into cancer cells. It is characterized by a progression of changes on cellular and genetic level that ultimately reprogram a cell to undergo uncontrolled cell division, thus forming a malignant mass.
Cell division is a physiological process that occurs in almost all tissues and under many circumstances. Under normal circumstances, the balance between proliferation and programmed cell death, usually in the form of apoptosis, is maintained by tightly regulating both processes to ensure the integrity of organs and tissues. Mutations in DNA that lead to cancer (only certain mutations can lead to cancer and the majority of potential mutations will have no bearing) disrupt these orderly processes by disrupting the programming regulating the processes.
Carcinogenesis is caused by this mutation of the genetic material of normal cells, which upsets the normal balance between proliferation and cell death. This results in uncontrolled cell division and the evolution of those cells by natural selection in the body. The uncontrolled and often rapid proliferation of cells can lead to benign tumors; some types of these may turn into malignant tumors (cancer). Benign tumors do not spread to other parts of the body or invade other tissues, and they are rarely a threat to life unless they compress vital structures or are physiologically active, for instance, producing a hormone. Malignant tumors can invade other organs, spread to distant locations (metastasis) and become life-threatening.
More than one mutation is necessary for carcinogenesis. In fact, a series of several mutations to certain classes of genes is usually required before a normal cell will transform into a cancer cell.[1] Only mutations in those certain types of genes that play vital roles in cell division, apoptosis (cell death), and DNA repair will cause a cell to lose control of its cell proliferation.
Oncovirinae, retroviruses that contain an oncogene, are categorized as oncogenic because they trigger the growth of tumorous tissues in the host. This process is also referred to as viral transformation.
Cancer is fundamentally a disease of regulation of tissue growth. In order for a normal cell to transform into a cancer cell, genes that regulate cell growth and differentiation must be altered.[2] Genetic changes can occur at many levels, from gain or loss of entire chromosomes to a mutation affecting a single DNA nucleotide. There are two broad categories of genes that are affected by these changes. Oncogenes may be normal genes that are expressed at inappropriately high levels, or altered genes that have novel properties. In either case, expression of these genes promotes the malignant phenotype of cancer cells. Tumor suppressor genes are genes that inhibit cell division, survival, or other properties of cancer cells. Tumor suppressor genes are often disabled by cancer-promoting genetic changes. Typically, changes in many genes are required to transform a normal cell into a cancer cell.[3]
There is a diverse classification scheme for the various genomic changes that may contribute to the generation of cancer cells. Most of these changes are mutations, or changes in the nucleotide sequence of genomic DNA. Aneuploidy, the presence of an abnormal number of chromosomes, is one genomic change that is not a mutation, and may involve either gain or loss of one or more chromosomes through errors in mitosis.
Large-scale mutations involve the deletion or gain of a portion of a chromosome. Genomic amplification occurs when a cell gains many copies (often 20 or more) of a small chromosomal region, usually containing one or more oncogenes and adjacent genetic material. Translocation occurs when two separate chromosomal regions become abnormally fused, often at a characteristic location. A well-known example of this is the Philadelphia chromosome, or translocation of chromosomes 9 and 22, which occurs in chronic myelogenous leukemia, and results in production of the BCR-abl fusion protein, an oncogenic tyrosine kinase.
Small-scale mutations include point mutations, deletions, and insertions, which may occur in the promoter of a gene and affect its expression, or may occur in the gene's coding sequence and alter the function or stability of its protein product. Disruption of a single gene may also result from integration of genomic material from a DNA virus or retrovirus, and such an event may also result in the expression of viral oncogenes in the affected cell and its descendants.
Contents
Cause
It is impossible to determine the initial cause for most specific cancers. In a few cases, only one cause exists; for example, the virus HHV-8 causes all Kaposi's sarcomas. However, with the help of cancer epidemiology techniques and information, it is possible to produce an estimate of a likely cause in many more situations. For example, lung cancer has several causes, including tobacco use and radon gas. Men who currently smoke tobacco develop lung cancer at a rate 14 times that of men who have never smoked tobacco, so the chance of lung cancer in a current smoker being caused by smoking is about 93%; there is a 7% chance that the smoker's lung cancer was caused by radon gas or some other, non-tobacco cause.[4] These statistical correlations have made it possible for researchers to infer that certain substances or behaviors are carcinogenic.
Using molecular biological techniques, it is possible to characterize the mutations or chromosomal aberrations within a tumor, and rapid progress is being made in the field of predicting prognosis based on the spectrum of mutations in some cases. For example, up to half of all tumors have a defective p53 gene. This mutation is associated with poor prognosis, since those tumor cells are less likely to go into apoptosis or programmed cell death when damaged by therapy. Telomerase mutations remove additional barriers, extending the number of times a cell can divide. Other mutations enable the tumor to grow new blood vessels to provide more nutrients, or to metastasize, spreading to other parts of the body.
Non-mainstream theories
There are a number of theories of carcinogenesis and cancer treatment that fall outside the mainstream of scientific opinion, due to lack of scientific rationale, logic, or evidence base. These theories may be used to justify various alternative cancer treatments. They should be distinguished from those theories of carcinogenesis that have a logical basis within mainstream cancer biology, and from which conventionally-testable hypotheses can be made.
Several alternative theories of carcinogenesis, however, are based on scientific evidence and are increasingly being acknowledged. Some researchers believe that cancer may be caused by epigenetic alterations (heritable and reversible changes other than the DNA sequence)[5] or aneuploidy (numerical and structural abnormalities in chromosomes)[6] rather than by mutations. Cancer has also been considered as a metabolic disease in which the cellular metabolism of oxygen is diverted from the pathway that generates energy (oxidative phosphorylation) to the pathway that generates reactive oxygen species (figure). This causes an energy switch from oxidative phosphorylation to aerobic glycolysis (Warburg's hypothesis) and the accumulation of reactive oxygen species leading to oxidative stress (oxidative stress theory of cancer).[7] All these theories of carcinogenesis may be complementary rather than contradictory.
Another theory as to the origin of cancer was developed by astrobiologists and suggests that cancer is an atavism, an evolutionary throwback to an earlier form of multicellular life.[8] The genes responsible for uncontrolled cell growth and cooperation between cancer cells are very similar to those that enabled the first multicellular life forms to group together and flourish. These genes still exist within the genome of more complex metazoans, such as humans, although more recently evolved genes keep them in check. When the newer controlling genes fail for whatever reason, the cell can revert to its more primitive programming and reproduce out of control. The theory is an alternative to the notion that cancers begin with rogue cells that undergo evolution within the body. Instead they possess a fixed number of primitive genes that are progressively activated, giving them finite variability.[9]
Cancer cell biology
Often, the multiple genetic changes that result in cancer may take many years to accumulate. During this time, the biological behavior of the pre-malignant cells slowly change from the properties of normal cells to cancer-like properties. Pre-malignant tissue can have a distinctive appearance under the microscope. Among the distinguishing traits are an increased number of dividing cells, variation in nuclear size and shape, variation in cell size and shape, loss of specialized cell features, and loss of normal tissue organization. Dysplasia is an abnormal type of excessive cell proliferation characterized by loss of normal tissue arrangement and cell structure in pre-malignant cells. These early neoplastic changes must be distinguished from hyperplasia, a reversible increase in cell division caused by an external stimulus, such as a hormonal imbalance or chronic irritation.
The most severe cases of dysplasia are referred to as "carcinoma in situ." In Latin, the term "in situ" means "in place", so carcinoma in situ refers to an uncontrolled growth of cells that remains in the original location and has not shown invasion into other tissues. Nevertheless, carcinoma in situ may develop into an invasive malignancy and is usually removed surgically, if possible.
Clonal evolution
Main article: Somatic evolution in cancerJust like a population of animals undergoes evolution, an unchecked population of cells also can undergo evolution. This undesirable process is called somatic evolution, and is how cancer arises and becomes more malignant.[10]
Most changes in cellular metabolism that allow cells to grow in a disorderly fashion lead to cell death. However once cancer begins, cancer cells undergo a process of natural selection: the few cells with new genetic changes that enhance their survival or reproduction continue to multiply, and soon come to dominate the growing tumor, as cells with less favorable genetic change are out-competed.[11] This is exactly how pathogens such as MRSA can become antibiotic-resistant (or how HIV can become drug-resistant), and the same reason why crop blights and pests can become pesticide-resistant. This evolution is why cancer recurrences will have cells that have acquired cancer-drug resistance (or in some cases, resistance to radiation from radiotherapy).
Biological properties of cancer cells
 When normal cells are damaged beyond repair, they are eliminated by apoptosis (A). Cancer cells avoid apoptosis and continue to multiply in an unregulated manner (B).
When normal cells are damaged beyond repair, they are eliminated by apoptosis (A). Cancer cells avoid apoptosis and continue to multiply in an unregulated manner (B).
In a 2000 article by Hanahan and Weinberg, the biological properties of malignant tumor cells were summarized as follows:[12]
- Acquisition of self-sufficiency in growth signals, leading to unchecked growth.
- Loss of sensitivity to anti-growth signals, also leading to unchecked growth.
- Loss of capacity for apoptosis, in order to allow growth despite genetic errors and external anti-growth signals.
- Loss of capacity for senescence, leading to limitless replicative potential (immortality)
- Acquisition of sustained angiogenesis, allowing the tumor to grow beyond the limitations of passive nutrient diffusion.
- Acquisition of ability to invade neighbouring tissues, the defining property of invasive carcinoma.
- Acquisition of ability to build metastases at distant sites, the classical property of malignant tumors (carcinomas or others).
The completion of these multiple steps would be a very rare event without :
- Loss of capacity to repair genetic errors, leading to an increased mutation rate (genomic instability), thus accelerating all the other changes.
These biological changes are classical in carcinomas; other malignant tumors may not need to achieve them all. For example, tissue invasion and displacement to distant sites are normal properties of leukocytes; these steps are not needed in the development of leukemia. The different steps do not necessarily represent individual mutations. For example, inactivation of a single gene, coding for the p53 protein, will cause genomic instability, evasion of apoptosis and increased angiogenesis. Not all the cancer cells are dividing. Rather, a subset of the cells in a tumor, called cancer stem cells, replicate themselves and generate differentiated cells.[13]
Mechanisms
Cancer is a genetic disease: In order for cells to start dividing uncontrollably, genes that regulate cell growth must be damaged.[14] Proto-oncogenes are genes that promote cell growth and mitosis, whereas tumor suppressor genes discourage cell growth, or temporarily halt cell division to carry out DNA repair. Typically, a series of several mutations to these genes is required before a normal cell transforms into a cancer cell. This concept is sometimes termed "oncoevolution." Mutations to these genes provide the signals for tumor cells to start dividing uncontrollably. But the uncontrolled cell division that characterizes cancer also requires that the dividing cell duplicates all its cellular components to create two daughter cells. The activation of anaerobic glycolysis (the Warburg effect), which is not necessarily induced by mutations in proto-oncogenes and tumor suppressor genes,[15] provides most of the building blocks required to duplicate the cellular components of a dividing cell and, therefore, is also essential for carcinogenesis.[16]
Cell types involved in cancer growth
There are several different cell types that are critical to tumour growth. In particular endothelial progenitor cells are a very important cell population in tumour blood vessel growth.[17][18] This importance of endothelial progenitor cells in tumour growth, angiogenesis and metastasis has been confirmed by a recent publication in Cancer Research (August 2010). This seminal paper has demonstrated that endothelial progenitor cells can be marked using the Inhibitor of DNA Binding 1 (ID1). This novel finding meant that investigators were able to track endothelial progenitor cells from the bone marrow to the blood to the tumour-stroma and even incorporated in tumour vasculature. This finding of endothelial progenitor cells incorporated in tumour vasculature proves the importance of this cell type in blood vessel development in a tumour setting and metastasis. Furthermore, ablation of the endothelial progenitor cells in the bone marrow lead to a significant decrease in tumour growth and vasculature development. Therefore endothelial progenitor cells are very important in tumour biology and present novel therapeutic targets.[19]
Oncogenes
Oncogenes promote cell growth through a variety of ways. Many can produce hormones, a "chemical messenger" between cells that encourage mitosis, the effect of which depends on the signal transduction of the receiving tissue or cells. In other words, when a hormone receptor on a recipient cell is stimulated, the signal is conducted from the surface of the cell to the cell nucleus to affect some change in gene transcription regulation at the nuclear level. Some oncogenes are part of the signal transduction system itself, or the signal receptors in cells and tissues themselves, thus controlling the sensitivity to such hormones. Oncogenes often produce mitogens, or are involved in transcription of DNA in protein synthesis, which creates the proteins and enzymes responsible for producing the products and biochemicals cells use and interact with.
Mutations in proto-oncogenes, which are the normally quiescent counterparts of oncogenes, can modify their expression and function, increasing the amount or activity of the product protein. When this happens, the proto-oncogenes become oncogenes, and this transition upsets the normal balance of cell cycle regulation in the cell, making uncontrolled growth possible. The chance of cancer cannot be reduced by removing proto-oncogenes from the genome, even if this were possible, as they are critical for growth, repair and homeostasis of the organism. It is only when they become mutated that the signals for growth become excessive.
One of the first oncogenes to be defined in cancer research is the ras oncogene. Mutations in the Ras family of proto-oncogenes (comprising H-Ras, N-Ras and K-Ras) are very common, being found in 20% to 30% of all human tumours.[20] Ras was originally identified in the Harvey sarcoma virus genome, and researchers were surprised that not only is this gene present in the human genome but also, when ligated to a stimulating control element, it could induce cancers in cell line cultures.[21]
Proto-oncogenes
Proto-oncogenes promote cell growth in a variety of ways. Many can produce hormones, "chemical messengers" between cells that encourage mitosis, the effect of which depends on the signal transduction of the receiving tissue or cells. Some are responsible for the signal transduction system and signal receptors in cells and tissues themselves, thus controlling the sensitivity to such hormones. They often produce mitogens, or are involved in transcription of DNA in protein synthesis, which create the proteins and enzymes is responsible for producing the products and biochemicals cells use and interact with.
Mutations in proto-oncogenes can modify their expression and function, increasing the amount or activity of the product protein. When this happens, they become oncogenes, and, thus, cells have a higher chance to divide excessively and uncontrollably. The chance of cancer cannot be reduced by removing proto-oncogenes from the genome, as they are critical for growth, repair and homeostasis of the body. It is only when they become mutated that the signals for growth become excessive. It is important to note that a gene possessing a growth-promoting role may increase carcinogenic potential of a cell, under the condition that all necessary cellular mechanisms that permit growth are activated.[22] This condition includes also the inactivation of specific tumor suppressor genes (see below). If the condition is not fulfilled, the cell may cease to grow and can proceed to die. This makes knowledge of the stage and type of cancer cell that grows under the control of a given oncogene crucial for the development of treatment strategies.
Tumor suppressor genes
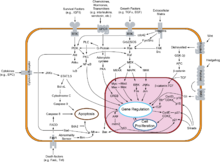 Many tumor suppressor genes effect signal transduction pathways that regulate apoptosis, also known as "programmed cell death".
Many tumor suppressor genes effect signal transduction pathways that regulate apoptosis, also known as "programmed cell death".
Tumor suppressor genes code for anti-proliferation signals and proteins that suppress mitosis and cell growth. Generally, tumor suppressors are transcription factors that are activated by cellular stress or DNA damage. Often DNA damage will cause the presence of free-floating genetic material as well as other signs, and will trigger enzymes and pathways that lead to the activation of tumor suppressor genes. The functions of such genes is to arrest the progression of the cell cycle in order to carry out DNA repair, preventing mutations from being passed on to daughter cells. The p53 protein, one of the most important studied tumor suppressor genes, is a transcription factor activated by many cellular stressors including hypoxia and ultraviolet radiation damage.
Despite nearly half of all cancers possibly involving alterations in p53, its tumor suppressor function is poorly understood. p53 clearly has two functions: one a nuclear role as a transcription factor, and the other a cytoplasmic role in regulating the cell cycle, cell division, and apoptosis.
The Warburg hypothesis is the preferential use of glycolysis for energy to sustain cancer growth. p53 has been shown to regulate the shift from the respiratory to the glycolytic pathway.[23]
However, a mutation can damage the tumor suppressor gene itself, or the signal pathway that activates it, "switching it off". The invariable consequence of this is that DNA repair is hindered or inhibited: DNA damage accumulates without repair, inevitably leading to cancer.
Mutations of tumor suppressor genes that occur in germline cells are passed along to offspring, and increase the likelihood for cancer diagnoses in subsequent generations. Members of these families have increased incidence and decreased latency of multiple tumors. The tumor types are typical for each type of tumor suppressor gene mutation, with some mutations causing particular cancers, and other mutations causing others. The mode of inheritance of mutant tumor suppressors is that an affected member inherits a defective copy from one parent, and a normal copy from the other. For instance, individuals who inherit one mutant p53 allele (and are therefore heterozygous for mutated p53) can develop melanomas and pancreatic cancer, known as Li-Fraumeni syndrome. Other inherited tumor suppressor gene syndromes include Rb mutations, linked to retinoblastoma, and APC gene mutations, linked to adenopolyposis colon cancer. Adenopolyposis colon cancer is associated with thousands of polyps in colon while young, leading to colon cancer at a relatively early age. Finally, inherited mutations in BRCA1 and BRCA2 lead to early onset of breast cancer.
Development of cancer was proposed in 1971 to depend on at least two mutational events. In what became known as the Knudson two-hit hypothesis, an inherited, germ-line mutation in a tumor suppressor gene would cause cancer only if another mutation event occurred later in the organism's life, inactivating the other allele of that tumor suppressor gene.[24]
Usually, oncogenes are dominant, as they contain gain-of-function mutations, while mutated tumor suppressors are recessive, as they contain loss-of-function mutations. Each cell has two copies of the same gene, one from each parent, and under most cases gain of function mutations in just one copy of a particular proto-oncogene is enough to make that gene a true oncogene. On the other hand, loss of function mutations need to happen in both copies of a tumor suppressor gene to render that gene completely non-functional. However, cases exist in which one mutated copy of a tumor suppressor gene can render the other, wild-type copy non-functional. This phenomenon is called the dominant negative effect and is observed in many p53 mutations.
Knudson’s two hit model has recently been challenged by several investigators. Inactivation of one allele of some tumor suppressor genes is sufficient to cause tumors. This phenomenon is called haploinsufficiency and has been demonstrated by a number of experimental approaches. Tumors caused by haploinsufficiency usually have a later age of onset when compared with those by a two hit process.[25]
Multiple mutations
In general, mutations in both types of genes are required for cancer to occur. For example, a mutation limited to one oncogene would be suppressed by normal mitosis control and tumor suppressor genes, first hypothesised by the Knudson hypothesis.[26] A mutation to only one tumor suppressor gene would not cause cancer either, due to the presence of many "backup" genes that duplicate its functions. It is only when enough proto-oncogenes have mutated into oncogenes, and enough tumor suppressor genes deactivated or damaged, that the signals for cell growth overwhelm the signals to regulate it, that cell growth quickly spirals out of control. Often, because these genes regulate the processes that prevent most damage to genes themselves, the rate of mutations increases as one gets older, because DNA damage forms a feedback loop.
Usually, oncogenes are dominant alleles, as they contain gain-of-function mutations, whereas mutated tumor suppressors are recessive alleles, as they contain loss-of-function mutations. Each cell has two copies of a same gene, one from each parent, and, under most cases, gain of function mutation in one copy of a particular proto-oncogene is enough to make that gene a true oncogene, while usually loss of function mutation must happen in both copies of a tumor suppressor gene to render that gene completely non-functional. However, cases exist in which one loss of function copy of a tumor suppressor gene can render the other copy non-functional, called the dominant negative effect. This is observed in many p53 mutations.
Mutation of tumor suppressor genes that are passed on to the next generation of not merely cells, but their offspring, can cause increased likelihoods for cancers to be inherited. Members within these families have increased incidence and decreased latency of multiple tumors. The mode of inheritance of mutant tumor suppressors is that affected member inherits a defective copy from one parent, and a normal copy from another. Because mutations in tumor suppressors act in a recessive manner (note, however, there are exceptions), the loss of the normal copy creates the cancer phenotype. For instance, individuals that are heterozygous for p53 mutations are often victims of Li-Fraumeni syndrome, and that are heterozygous for Rb mutations develop retinoblastoma. In similar fashion, mutations in the adenomatous polyposis coli gene are linked to adenopolyposis colon cancer, with thousands of polyps in the colon while young, whereas mutations in BRCA1 and BRCA2 lead to early onset of breast cancer.
A new idea announced in 2011 is an extreme version of multiple mutations, called chromothripsis by its proponents. This idea, affecting only 2–3% of cases of cancer, although up to 25% of bone cancers, involves the catastrophic shattering of a chromosome into tens or hundreds of pieces and then being patched back together incorrectly. This shattering probably takes place when the chromosomes are compacted during normal cell division, but the trigger for the shattering is unknown. Under this model, cancer arises as the result of a single, isolated event, rather than the slow accumulation of multiple mutations.[27]
Non-mutagenic carcinogens
Many mutagens are also carcinogens, but some carcinogens are not mutagens. Examples of carcinogens that are not mutagens include alcohol and estrogen. These are thought to promote cancers through their stimulating effect on the rate of cell mitosis. Faster rates of mitosis increasingly leave fewer opportunities for repair enzymes to repair damaged DNA during DNA replication, increasing the likelihood of a genetic mistake. A mistake made during mitosis can lead to the daughter cells' receiving the wrong number of chromosomes, which leads to aneuploidy and may lead to cancer.
Role of infections
Bacterial
Main article: Cancer bacteriaHeliobacter pylori is known to cause MALT lymphoma. Other types of bacteria have been implicated in other cancers.
Viral
Main article: OncovirusFurthermore, many cancers originate from a viral infection; this is especially true in animals such as birds, but less so in humans. 12% of human cancers can be attributed to a viral infection.[28] The mode of virally-induced tumors can be divided into two, acutely-transforming or slowly-transforming. In acutely-transforming viruses, the viral particles carry a gene that encodes for an overactive oncogene called viral-oncogene (v-onc), and the infected cell is transformed as soon as v-onc is expressed. In contrast, in slowly-transforming viruses, the virus genome is inserted, especially as viral genome insertion is obligatory part of retroviruses, near a proto-oncogene in the host genome. The viral promoter or other transcription regulation elements, in turn, cause over-expression of that proto-oncogene, which, in turn, induces uncontrolled cellular proliferation. Because viral genome insertion is not specific to proto-oncogenes and the chance of insertion near that proto-oncogene is low, slowly-transforming viruses have very long tumor latency compared to acutely-transforming virus, which already carries the viral-oncogene.
Viruses that are known to cause cancer such as HPV (cervical cancer), Hepatitis B (liver cancer), and EBV (a type of lymphoma), are all DNA viruses. It is thought that when the virus infects a cell, it inserts a part of its own DNA near the cell growth genes, causing cell division. The group of changed cells that are formed from the first cell dividing all have the same viral DNA near the cell growth genes. The group of changed cells are now special because one of the normal controls on growth has been lost.
Depending on their location, cells can be damaged through radiation from sunshine, chemicals from cigarette smoke, and inflammation from bacterial infection or other viruses. Each cell has a chance of damage, a step on a path toward cancer. Cells often die if they are damaged, through failure of a vital process or the immune system; however, sometimes damage will knock out a single cancer gene. In an old person, there are thousands, tens of thousands or hundreds of thousands of knocked-out cells. The chance that any one would form a cancer is very low.
When the damage occurs in any area of changed cells, something different occurs. Each of the cells has the potential for growth. The changed cells will divide quicker when the area is damaged by physical, chemical, or viral agents. A vicious circle has been set up: Damaging the area will cause the changed cells to divide, causing a greater likelihood that they will suffer knock-outs.
This model of carcinogenesis is popular because it explains why cancers grow. It would be expected that cells that are damaged through radiation would die or at least be worse off because they have fewer genes working; viruses increase the number of genes working.
One concern is that we may end up with thousands of vaccines to prevent every virus that can change our cells. Viruses can have different effects on different parts of the body. It may be possible to prevent a number of different cancers by immunizing against one viral agent. It is likely that HPV, for instance, has a role in cancers of the mucous membranes of the mouth.
Helminthiasis
Certain parasitic worms are known to be carcinogenic.[29] These include:
- Clonorchis sinensis (the organism causing Clonorchiasis) and Opisthorchis viverrini (causing Opisthorchiasis) are associated with cholangiocarcinoma.[30]
- Schistosoma species (the organisms causing Schistosomiasis) is associated with bladder cancer.
Epigenetics
Epigenetics is the study of the regulation of gene expression through chemical, non-mutational changes in DNA structure. The theory of epigenetics in cancer pathogenesis is that non-mutational changes to DNA can lead to alterations in gene expression. Normally, oncogenes are silent, for example, because of DNA methylation. Loss of that methylation can induce the aberrant expression of oncogenes, leading to cancer pathogenesis. Known mechanisms of epigenetic change include DNA methylation, and methylation or acetylation of histone proteins bound to chromosomal DNA at specific locations. Classes of medications, known as HDAC inhibitors and DNA methyltransferase inhibitors, can re-regulate the epigenetic signaling in the cancer cell.
Cancer stem cells
Main article: Cancer stem cellA new way of looking at carcinogenesis comes from integrating the ideas of developmental biology into oncology. The cancer stem cell hypothesis proposes that the different kinds of cells in a heterogeneous tumor arise from a single cell, termed Cancer Stem Cell. Cancer stem cells may arise from transformation of adult stem cells or differentiated cells within a body. These cells persist as a subcomponent of the tumor and retain key stem cell properties. They give rise to a variety of cells, are capable of self-renewal and homeostatic control.[31] Furthermore, the relapse of cancer and the emergence of metastasis are also attributed to these cells. The cancer stem cell hypothesis does not contradict earlier concepts of carcinogenesis.
Clonal evolution
Main article: Somatic evolution in cancerWhile genetic and epigenetic alterations in tumor suppressor genes and oncogenes change the behavior of cells, those alterations, in the end, result in cancer through their effects on the population of neoplastic cells and their microenvironment.[32] Mutant cells in neoplasms compete for space and resources. Thus, a clone with a mutation in a tumor suppressor gene or oncogene will expand only in a neoplasm if that mutation gives the clone a competitive advantage over the other clones and normal cells in its microenvironment.[33] Thus, the process of carcinogenesis is formally a process of Darwinian evolution, known as somatic or clonal evolution.[34] Furthermore, in light of the Darwinistic mechanisms of carcinogenesis, it has been theorized that the various forms of cancer can be categorized as pubertarial and gerontological. Anthropological research is currently being conducted on cancer as a natural evolutionary process through which natural selection destroys environmentally inferior phenotypes while supporting others. According to this theory, cancer comes in two separate types: from birth to the end of puberty (approximately age 20) teleologically inclined toward supportive group dynamics, and from mid-life to death (approximately age 40+) teleologically inclined away from overpopulative group dynamics.
References
- ^ Fearon ER, Vogelstein B (June 1990). "A genetic model for colorectal tumorigenesis". Cell 61 (5): 759–67. doi:10.1016/0092-8674(90)90186-I. PMID 2188735.
- ^ Croce CM (January 2008). "Oncogenes and cancer". The New England journal of medicine 358 (5): 502–11. doi:10.1056/NEJMra072367. PMID 18234754. http://content.nejm.org/cgi/content/full/358/5/502.
- ^ Knudson AG (November 2001). "Two genetic hits (more or less) to cancer". Nature reviews. Cancer 1 (2): 157–62. doi:10.1038/35101031. PMID 11905807.
- ^ Villeneuve, PJ; Mao Y (November 1994). "Lifetime probability of developing lung cancer, by smoking status, Canada". Canadian Journal of Public Health 85 (6): 385–388. PMID 7895211.
- ^ Jaffe, LF (2003). "Epigenetic theories of cancer initiation". Advances in cancer research 90: 209–30. doi:10.1016/S0065-230X(03)90007-8. PMID 14710952.
- ^ Rasnick, D; Duesberg, PH (1999). "How aneuploidy affects metabolic control and causes cancer". The Biochemical journal 340: 621–30. doi:10.1042/0264-6021:3400621. PMC 1220292. PMID 10359645. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1220292.
- ^ López-Lázaro, M (2010). "A new view of carcinogenesis and an alternative approach to cancer therapy". Molecular medicine 16 (3-4): 144–153. doi:10.2119/molmed.2009.00162. PMC 2802554. PMID 20062820. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2802554.
- ^ P C W Davies and C H Lineweaver (February 2011). "Cancer tumors as Metazoa 1.0: tapping genes of ancient ancestors". Phys. Biol. 8 (1): 1–7. doi:10.1088/1478-3975/8/1/015001.
- ^ Dean, Tim. "Cancer resembles life 1 billion years ago, say astrobiologists", Australian Life Scientist, 08 February 2011. Retrieved on 2011-02-15.
- ^ Nowell PC (October 1976). "The clonal evolution of tumor cell populations". Science 194 (4260): 23–8. doi:10.1126/science.959840. PMID 959840. http://www.sciencemag.org/cgi/pmidlookup?view=long&pmid=959840.
- ^ Merlo LM, Pepper JW, Reid BJ, Maley CC (December 2006). "Cancer as an evolutionary and ecological process". Nat Rev Cancer 6 (12): 924–35. doi:10.1038/nrc2013. PMID 17109012.
- ^ Hanahan D, Weinberg RA (2000). "The hallmarks of cancer". Cell 100 (1): 57–70. doi:10.1016/S0092-8674(00)81683-9. PMID 10647931.
- ^ Cho RW, Clarke MF (February 2008). "Recent advances in cancer stem cells". Curr. Opin. Genet. Dev. 18 (1): 48–53. doi:10.1016/j.gde.2008.01.017. PMID 18356041.
- ^ Vogelstein, Bert; Kinzler, Kenneth W (2004). "Cancer genes and the pathways they control". Nature Medicine 10 (8): 789–99. doi:10.1038/nm1087. PMID 15286780.
- ^ Brand, KA; Hermfisse, U (1997). "Aerobic glycolysis by proliferating cells: a protective strategy against reactive oxygen species". The FASEB journal 11 (5): 388–95. PMID 9141507.
- ^ López-Lázaro, M (2010). "A new view of carcinogenesis and an alternative approach to cancer therapy". Molecular medicine 16 (3-4): 144–153. doi:10.2119/molmed.2009.00162. PMC 2802554. PMID 20062820. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2802554.
- ^ Gao D et al. (2008). "Endothelial Progenitor Cells Control the Angiogenic Switch in Mouse Lung Metastasis". Science 319 (5860): 195–198. doi:10.1126/science.1150224. PMID 18187653.
- ^ Nolan DJ et al. (2007). "Bone marrow-derived endothelial progenitor cells are a major determinant of nascent tumor neovascularization". Genes and Development 21 (12): 1546–1558. doi:10.1101/gad.436307. PMC 1891431. PMID 17575055. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1891431.
- ^ Mellick As, Plummer PN et al. (2010). "Using the Transcription Factor Inhibitor of DNA Binding 1 to Selectively Target Endothelial Progenitor Cells Offers Novel Strategies to Inhibit Tumor Angiogenesis and Growth". Cancer Research 70 (18): 7273–7282. doi:10.1158/0008-5472.CAN-10-1142. PMC 3058751. PMID 20807818. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3058751.
- ^ Bos JL (September 1989). "ras oncogenes in human cancer: a review". Cancer Research 49 (17): 4682–9. PMID 2547513. http://cancerres.aacrjournals.org/cgi/pmidlookup?view=long&pmid=2547513. Retrieved 2009-06-06.
- ^ Chang EH, Furth ME, Scolnick EM, Lowy DR (1982). "Tumorigenic transformation of mammalian cells induced by a normal human gene homologous to the oncogene of Harvey murine sarcoma virus". Nature 297 (5866): 479–83. doi:10.1038/297479a0. PMID 6283358.
- ^ Vlahopoulos SA, Logotheti S, Mikas D, Giarika A, Gorgoulis V, Zoumpourlis V (April 2008). "The role of ATF-2 in oncogenesis". BioEssays 30 (4): 314–27. doi:10.1002/bies.20734. PMID 18348191.
- ^ Matoba S, Kang J, Patino W, Wragg A, Boehm M, Gavrilova O, Hurley P, Bunz F, Hwang P (2006). "p53 regulates mitochondrial respiration". Science 312 (5780): 1650–3. doi:10.1126/science.1126863. PMID 16728594.
- ^ Knudson A (1971). "Mutation and cancer: statistical study of retinoblastoma". Proc Natl Acad Sci USA 68 (4): 820–3. doi:10.1073/pnas.68.4.820. PMC 389051. PMID 5279523. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=389051.
- ^ Fodde R, Smits R (2002). "Cancer biology. A matter of dosage". Science 298 (5594): 761–3. doi:10.1126/science.1077707. PMID 12399571.
- ^ Knudson AG (November 2001). "Two genetic hits (more or less) to cancer". Nature Reviews. Cancer 1 (2): 157–62. doi:10.1038/35101031. PMID 11905807.
- ^ Stephens PJ, Greenman CD, Fu B, et al. (January 2011). "Massive Genomic Rearrangement Acquired in a Single Catastrophic Event during Cancer Development". Cell 144 (1): 27–40. doi:10.1016/j.cell.2010.11.055. PMC 3065307. PMID 21215367. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3065307. Lay summary – The New York times (10 January 2011).
- ^ Carrillo-Infante C, Abbadessa G, Bagella L, Giordano A (June 2007). "Viral infections as a cause of cancer (review)". Int. J. Oncol. 30 (6): 1521–8. PMID 17487374. http://www.spandidos-publications.com/ijo/article.jsp?article_id=ijo_30_6_1521.
- ^ Safdar, Amar (2011-06-01). Management of Infections in Cancer Patients. Springer. pp. 478–. ISBN 9781607616436. http://books.google.com/books?id=TpWhwLCDWWsC&pg=PA478. Retrieved 17 August 2011.
- ^ Samaras V, Rafailidis PI, Mourtzoukou EG, Peppas G, Falagas ME (May 2010). "Chronic bacterial and parasitic infections and cancer: a review". J Infect Dev Ctries 4 (5): 267–81. PMID 20539059. http://www.jidc.org/index.php/journal/article/view/20539059.
- ^ Dalerba, P.; Cho, R. W.; Clarke, M. F. (2007). "Cancer stem cells: models and concepts". Annu. Rev. Med. 58: 267–284. doi:10.1146/annurev.med.58.062105.204854. PMID 17002552.
- ^ Nowell PC (October 1976). "The clonal evolution of tumor cell populations". Science 194 (4260): 23–8. doi:10.1126/science.959840. PMID 959840.
- ^ Zhang W, Hanks AN, Boucher K et al. (January 2005). "UVB-induced apoptosis drives clonal expansion during skin tumor development". Carcinogenesis 26 (1): 249–57. doi:10.1093/carcin/bgh300. PMC 2292404. PMID 15498793. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2292404. Retrieved 2009-06-19.
- ^ Merlo LM, Pepper JW, Reid BJ, Maley CC (December 2006). "Cancer as an evolutionary and ecological process". Nature Reviews. Cancer 6 (12): 924–35. doi:10.1038/nrc2013. PMID 17109012.
Radoslav S.Jovic-Cancer-released ancestor from our genes,www.newcancertheory.com
Further reading
- Tokar, Erik J.; Benbrahim-Tallaa, Lamia; Waalkes, Michael P. (2011). "Chepter 14. Metal Ions in Human Cancer Development". In Astrid Sigel, Helmut Sigel and Roland K. O. Sigel. Metal ions in toxicology: effects, interactions, interdependencies. Metal Ions in Life Sciences. 8. RSC Publishing. pp. 375–401. doi:10.1039/9781849732116-00375.
- Dixon K, Kopras E (2004). "Genetic alterations and DNA repair in human carcinogenesis.". Semin Cancer Biol 14 (6): 441–8. doi:10.1016/j.semcancer.2004.06.007. PMID 15489137.
- Kleinsmith, Lewis J (2006). Principles of cancer biology. San Francisco: Pearson Benjamin Cummings. ISBN 978-0-8053-4003-7.
- Sarasin A (2003). "An overview of the mechanisms of mutagenesis and carcinogenesis.". Mutat Res 544 (2-3): 99–106. doi:10.1016/j.mrrev.2003.06.024. PMID 14644312.
- Schottenfeld D, Beebe-Dimmer JL (2005). "Advances in cancer epidemiology: understanding causal mechanisms and the evidence for implementing interventions.". Annu Rev Public Health 26: 37–60. doi:10.1146/annurev.publhealth.26.021304.144402. PMID 15760280.
- Tannock, Ian; Hill, Richard; Bristow, Robert; Harrington, Lea (2005). The basic science of oncology (4th ed.). New York: McGraw-Hill. ISBN 978-0-07-138774-3.
- Wicha MS, Liu S, Dontu G (2006). "Cancer stem cells: an old idea--a paradigm shift.". Cancer Res 66 (4): 1883–90. doi:10.1158/0008-5472.CAN-05-3153. PMID 16488983.
Pathology: Tumor, Neoplasm, Cancer, and Oncology (C00–D48, 140–239) Conditions Malignant progressionTopographyHead/Neck (Oral, Nasopharyngeal) · Digestive system · Respiratory system · Bone · Skin · Blood · Urogenital · Nervous system · Endocrine systemHistologyOtherPrecancerous condition · Paraneoplastic syndromeStaging/grading Carcinogenesis Misc. M: NEO
tsoc, mrkr
tumr, epon, para
drug (L1i/1e/V03)
Categories:- Carcinogenesis
- Radiation health effects
- Carcinogens
Wikimedia Foundation. 2010.


