- Schistosomiasis
-
Schistosomiasis Classification and external resources 
Skin vesicles on the forearm, created by the penetration of Schistosoma. Source: CDCICD-10 B65 ICD-9 120 MeSH D012552 Schistosomiasis (also known as bilharzia, bilharziosis or snail fever) is a parasitic disease caused by several species of trematodes (platyhelminth infection, or "flukes"), a parasitic worm of the genus Schistosoma. Snails often act as an intermediary agent for the infectious diseases until a new human host is found. Individuals within developing countries who cannot afford proper sanitation facilities are often exposed to contaminated water that contains the Schistosomiasis parasite. [1]
Although it has a low mortality rate, schistosomiasis often is a chronic illness that can damage internal organs and, in children, impair growth and cognitive development. The urinary form of schistosomiasis is associated with increased risks for bladder cancer in adults. Schistosomiasis is the second most socioeconomically devastating parasitic disease after malaria.[2]
This disease is most commonly found in Asia, Africa, and South America, especially in areas where the water contains numerous freshwater snails, which may carry the parasite.
The disease affects many people in developing countries, particularly children who may acquire the disease by swimming or playing in infected water.[2] As children come into contact with the contaminated water source the parasitic snail larva easily enter through the human skin and further mature within organ tissues. As of 2009, 74 developing countries statistically identified epidemics of Schistosomiasis within their respective populations. [3]
Contents
Classification
Species of Schistosoma that can infect humans:
- Schistosoma mansoni (ICD-10 B65.1) and Schistosoma intercalatum (B65.8) cause intestinal schistosomiasis
- Schistosoma haematobium (B65.0) causes urinary schistosomiasis
- Schistosoma japonicum (B65.2) and Schistosoma mekongi (B65.8) cause Asian intestinal schistosomiasis
Avian schistosomiasis species cause swimmer's itch and clam digger itch
Species of Schistosoma that can infect other animals:
S. bovis — normally infects cattle, sheep and goats in Africa, parts of Southern Europe and the Middle East
S. mattheei — normally infects cattle, sheep and goats in Central and Southern Africa
S. margrebowiei — normally infects antelope, buffalo and waterbuck in Southern and Central Africa
S. curassoni — normally infects domestic ruminants in West Africa
S. rodhaini — normally infects rodents and carnivores in parts of Central AfricaSigns and symptoms
Above all, schistosomiasis is a chronic disease. Many infections are subclinically symptomatic, with mild anemia and malnutrition being common in endemic areas. Acute schistosomiasis (Katayama's fever) may occur weeks after the initial infection, especially by S. mansoni and S. japonicum. Manifestations include:
- Abdominal pain
- Cough
- Diarrhea
- Eosinophilia — extremely high eosinophil granulocyte (white blood cell) count.
- Fever
- Fatigue
- Hepatosplenomegaly — the enlargement of both the liver and the spleen. Hepatic schistosomiasis is the second most common cause of esophageal varices[4] worldwide.
- Genital sores — lesions that increase vulnerability to HIV infection. Lesions caused by schistosomiasis may continue to be a problem after control of the schistosomiasis infection itself. Early treatment, especially of children, which is relatively inexpensive, prevents formation of the sores.[5][6]
- Skin symptoms: At the start of infection, mild itching and a papular dermatitis of the feet and other parts after swimming in polluted streams containing cercariae.[7]:432
Occasionally central nervous system lesions occur: cerebral granulomatous disease may be caused by ectopic S. japonicum eggs in the brain, and granulomatous lesions around ectopic eggs in the spinal cord from S. mansoni and S. haematobium infections may result in a transverse myelitis with flaccid paraplegia.
Continuing infection may cause granulomatous reactions and fibrosis in the affected organs, which may result in manifestations that include:
- Colonic polyposis with bloody diarrhea (Schistosoma mansoni mostly);
- Portal hypertension with hematemesis and splenomegaly (S. mansoni, S. japonicum);
- Cystitis and ureteritis (S. haematobium) with hematuria, which can progress to bladder cancer;
- Pulmonary hypertension (S. mansoni, S. japonicum, more rarely S. haematobium);
- Glomerulonephritis; and central nervous system lesions.
Bladder cancer diagnosis and mortality are generally elevated in affected areas.
Pathophysiology
Life cycle
Schistosomes have a typical trematode vertebrate-invertebrate lifecycle, with humans being the definitive host.
Snails
The life cycles of all five human schistosomes are broadly similar: parasite eggs are released into the environment from infected individuals, hatching on contact with fresh water to release the free-swimming miracidium. Miracidia infect fresh-water snails by penetrating the snail's foot. After infection, close to the site of penetration, the miracidium transforms into a primary (mother) sporocyst. Germ cells within the primary sporocyst will then begin dividing to produce secondary (daughter) sporocysts, which migrate to the snail's hepatopancreas. Once at the hepatopancreas, germ cells within the secondary sporocyst begin to divide again, this time producing thousands of new parasites, known as cercariae, which are the larvae capable of infecting mammals.
Cercariae emerge daily from the snail host in a circadian rhythm, dependent on ambient temperature and light. Young cercariae are highly mobile, alternating between vigorous upward movement and sinking to maintain their position in the water. Cercarial activity is particularly stimulated by water turbulence, by shadows and by chemicals found on human skin.
Humans
Penetration of the human skin occurs after the cercaria have attached to and explored the skin. The parasite secretes enzymes that break down the skin's protein to enable penetration of the cercarial head through the skin. As the cercaria penetrates the skin it transforms into a migrating schistosomulum stage.
The newly transformed schistosomulum may remain in the skin for 2 days before locating a post-capillary venule; from here the schistosomulum travels to the lungs where it undergoes further developmental changes necessary for subsequent migration to the liver. Eight to ten days after penetration of the skin, the parasite migrates to the liver sinusoids. S. japonicum migrates more quickly than S. mansoni, and usually reaches the liver within 8 days of penetration. Juvenile S. mansoni and S. japonicum worms develop an oral sucker after arriving at the liver, and it is during this period that the parasite begins to feed on red blood cells. The nearly-mature worms pair, with the longer female worm residing in the gynaecophoric channel of the shorter male. Adult worms are about 10 mm long. Worm pairs of S. mansoni and S. japonicum relocate to the mesenteric or rectal veins. S. haematobium schistosomula ultimately migrate from the liver to the perivesical venous plexus of the bladder, ureters, and kidneys through the hemorrhoidal plexus.
Parasites reach maturity in six to eight weeks, at which time they begin to produce eggs. Adult S. mansoni pairs residing in the mesenteric vessels may produce up to 300 eggs per day during their reproductive lives. S. japonicum may produce up to 3000 eggs per day. Many of the eggs pass through the walls of the blood vessels, and through the intestinal wall, to be passed out of the body in feces. S. haematobium eggs pass through the ureteral or bladder wall and into the urine. Only mature eggs are capable of crossing into the digestive tract, possibly through the release of proteolytic enzymes, but also as a function of host immune response, which fosters local tissue ulceration. Up to half the eggs released by the worm pairs become trapped in the mesenteric veins, or will be washed back into the liver, where they will become lodged. Worm pairs can live in the body for an average of four and a half years, but may persist up to 20 years.
Trapped eggs mature normally, secreting antigens that elicit a vigorous immune response. The eggs themselves do not damage the body. Rather it is the cellular infiltration resultant from the immune response that causes the pathology classically associated with schistosomiasis.
Diagnosis
Microscopic identification of eggs in stool or urine is the most practical method for diagnosis. The stool exam is the more common of the two. For the measurement of eggs in the feces of presenting patients the scientific unit used is eggs per gram (epg). Stool examination should be performed when infection with S. mansoni or S. japonicum is suspected, and urine examination should be performed if S. haematobium is suspected.
Eggs can be present in the stool in infections with all Schistosoma species. The examination can be performed on a simple smear (1 to 2 mg of fecal material). Since eggs may be passed intermittently or in small amounts, their detection will be enhanced by repeated examinations and/or concentration procedures (such as the formalin-ethyl acetate technique). In addition, for field surveys and investigational purposes, the egg output can be quantified by using the Kato-Katz technique (20 to 50 mg of fecal material) or the Ritchie technique.
Eggs can be found in the urine in infections with S. japonicum and with S. intercalatum (recommended time for collection: between noon and 3 PM). Detection will be enhanced by centrifugation and examination of the sediment. Quantification is possible by using filtration through a nucleopore membrane of a standard volume of urine followed by egg counts on the membrane. Investigation of S. haematobium should also include a pelvic x-ray as bladder wall calcificaition is highly characteristic of chronic infection.
Recently a field evaluation of a novel handheld microscope was undertaken in Uganda for the diagnosis of intestinal schistosomiasis by a team led by Dr. Russell Stothard from the Natural History Museum of London, working with the Schistosomiasis Control Initiative, London.[8]
Tissue biopsy (rectal biopsy for all species and biopsy of the bladder for S. haematobium) may demonstrate eggs when stool or urine examinations are negative.
The eggs of S. haematobium are ellipsoidal with a terminal spine, S. mansoni eggs are also ellipsoidal but with a lateral spine, S. japonicum eggs are spheroidal with a small knob.
Antibody detection can be useful in both clinical management and for epidemiologic surveys.
Prevention
Eliminating or avoiding the snails
Prevention is best accomplished by eliminating the water-dwelling snails that are the natural reservoir of the disease. Acrolein, copper sulfate, and niclosamide can be used for this purpose. Recent studies have suggested that snail populations can be controlled by the introduction of, or augmentation of existing, crayfish populations; as with all ecological interventions, however, this technique must be approached with caution.
In 1989, Aklilu Lemma and Legesse Wolde-Yohannes received the Right Livelihood Award for their research on the sarcoca plant, as a preventative measure for the disease by controlling the snail. Concurrently, Dr Chidzere of Zimbabwe researched the similar gopo berry during the 1980s and found that it could be used in the control of infected freshwater snails. In 1989 he drew attention to his concerns that big chemical companies denigrated the gopo berry alternative for snail control.[9] Gopo berries from hotter Ethiopia climates reputedly yield the best results. Later studies were conducted between 1993 and 1995 by the Danish Research Network for international health.[10][11] For many years from the 1950s onwards, civil engineers built vast dam and irrigation schemes, oblivious to the fact that they would cause a massive rise in water-borne infections from schistosomiasis. The detailed specifications laid out in various UN documents since the 1950s could have minimized this problem. Irrigation schemes can be designed to make it hard for the snails to colonize the water, and to reduce the contact with the local population.[12]
This has been cited as a classic case of the relevance paradox because guidelines on how to design these schemes to minimise the spread of the disease had been published years before, but the designers were unaware of them.[13]
Treatment
Main article: SchistosomicideSchistosomiasis is readily treated using a single oral dose of the drug praziquantel annually.[14] As with other major parasitic diseases, there is ongoing and extensive research into developing a schistosomiasis vaccine that will prevent the parasite from completing its life cycle in humans. In 2009, Eurogentec Biologics developed a vaccine against bilharziosis in partnership with INSERM and researchers from the Pasteur Institute.[15][16][17]
The World Health Organization has developed guidelines for community treatment of schistosomiasis based on the impact the disease has on children in endemic villages:[14]
- When a village reports more than 50 percent of children have blood in their urine, everyone in the village receives treatment.[14]
- When 20 to 50 percent of children have bloody urine, only school-age children are treated.[14]
- When less than 20 percent of children have symptoms, mass treatment is not implemented.[14]
The Bill & Melinda Gates Foundation has recently funded an operational research program---the Schistosomiasis Consortium for Operational Research and Evaluation (SCORE) to answer strategic questions about how to move forward with schistosomiasis control and elimination. The focus of SCORE is on development of tools and evaluation of strategies for use in mass drug administration campaigns.
Antimony has been used in the past to treat the disease. In low doses, this toxic metalloid bonds to sulfur atoms in enzymes used by the parasite and kills it without harming the host. This treatment is not referred to in present-day peer-review scholarship; praziquantel is universally used. Outside of the U.S., there is a drug available exclusively for treating Schistosoma mansoni (oxamniquine) and one exclusively for treating S.hematobium (metrifonate). While metrifonate has been discontinued for use by the British National Health Service, a Cochrane review found it equally effective in treating urinary schistosomiasis as the leading drug, praziquantel.[18]
Mirazid, an Egyptian drug made from myrrh, was under investigation for oral treatment of the disease up until 2005.[19] The efficacy of praziquantel was proven to be about 8 times than that of Mirazid and therefore Mirazid was not recommended as a suitable agent to control schistosomiasis.[20]
Epidemiology
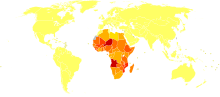 Disability-adjusted life year for schistosomiasis per 100,000 inhabitants.
Disability-adjusted life year for schistosomiasis per 100,000 inhabitants. no dataless than 5050-7575-100100-150150-200200-250250-300300-350350-400400-450450-500more than 500
no dataless than 5050-7575-100100-150150-200200-250250-300300-350350-400400-450450-500more than 500The disease is found in tropical countries in Africa, the Caribbean, eastern South America, Southeast Asia and in the Middle East. Schistosoma mansoni is found in parts of South America and the Caribbean, Africa, and the Middle East; S. haematobium in Africa and the Middle East; and S. japonicum in the Far East. S. mekongi and S. intercalatum are found locally in Southeast Asia and central West Africa, respectively.
Among human parasitic diseases, schistosomiasis (sometimes called bilharziasis) ranks second behind malaria in terms of socio-economic and public health importance in tropical and subtropical areas. The disease is endemic in 74-76[verification needed] developing countries, infecting more than 200 million people, half of whom live in Africa.[2] They live in rural agricultural and peri-urban areas, and placing more than 600 million people at risk.[21]
Of the infected patients, 20 million suffer severe consequences from the disease.[22] Some estimate that there are approximately 20,000 deaths related to schistosomiasis yearly.[citation needed] In many areas, schistosomiasis infects a large proportion of children under 14 years of age. An estimated 600 million people worldwide are at risk from the disease.
A few countries have eradicated the disease, and many more are working toward it.[citation needed] The World Health Organization is promoting these efforts. In some cases, urbanization, pollution, and/or consequent destruction of snail habitat has reduced exposure, with a subsequent decrease in new infections. The most common way of getting schistosomiasis in developing countries is by wading or swimming in lakes, ponds and other bodies of water that are infested with the snails (usually of the genera Biomphalaria, Bulinus, or Oncomelania) that are the natural reservoirs of the Schistosoma pathogen.
History
Schistosomiasis is known as bilharzia or bilharziosis in many countries, after Theodor Bilharz, who first described the cause of urinary schistosomiasis in 1851.
The first doctor who described the entire disease cycle was Pirajá da Silva in 1908.
It was a common cause of death for Ancient Egyptians in the Greco-Roman Period.
Society and culture
Egypt treatment campaign and Hepatitis C
Schistosomiasis is endemic in Egypt, exacerbated by the country's dam and irrigation projects along the Nile. From the late 1950s through the early 1980s, infected villagers were treated with repeated shots of tartar emetic. Epidemiological evidence suggests that this campaign unintentionally contributed to the spread of the hepatitis C virus via unclean needles. Egypt has the world's highest hepatitis C infection rate, and the infection rates in various regions of the country closely track the timing and intensity of the anti-schistosomiasis campaign.[23]
See also
- Genital schistosomiasis
- Tropical disease
- Male menstruation, a misunderstood symptom caused by schistosomiasis
References
- ^ "CIA - The World Factbook." Central Intelligence Agency, 4 Apr. 2007 {{cite web|url=https://www.cia.gov/library/publications/the-world-factbook/docs/notesanddefs.html#2193
- ^ a b c The Carter Center. "Schistosomiasis Control Program". http://www.cartercenter.org/health/schistosomiasis/index.html. Retrieved 2008-07-17
- ^ "CIA - The World Factbook." Central Intelligence Agency, 4 Apr. 2007 {{cite web|url=https://www.cia.gov/library/publications/the-world-factbook/docs/notesanddefs.html#2193
- ^ p.771 Robbin and Cotran Pathological Basis of Disease 8th
- ^ Donald G. McNeil, Jr. (May 25, 2009). "Parasites: Giving a Deworming Drug to Girls Could Cut H.I.V. Transmission in Africa". The New York Times. http://www.nytimes.com/2009/05/26/health/26glob.html.
- ^ Hotez PJ, Fenwick A, Kjetland EF (2009). "Africa's 32 Cents Solution for HIV/AIDS". PLoS Negl Trop Dis 3 (5): e430. doi:10.1371/journal.pntd.0000430. PMC 2682705. PMID 19479041. http://www.plosntds.org/article/info%3Adoi%2F10.1371%2Fjournal.pntd.0000430.
- ^ James, William D.; Berger, Timothy G.; et al. (2006). Andrews' Diseases of the Skin: clinical Dermatology. Saunders Elsevier. ISBN 0-7216-2921-0.
- ^ Stothard, J. Russell; et al. (2005). "Field Evaluation of the Meade Readview Handheld Microscope for Diagnosis of Intestinal Schistosomiasis in Ugandan Sschool Children". Am. J. Trop. Med. Hyg. (American Society of Tropical Medicine and Hygiene) 73 (5): 949–955. PMID 16282310. http://looksmall.com/images/stothardabstract.pdf.
- ^ The Gopu Berry p33. Part 4 School Journal number.2 1989 Dept of Education Wellington N.Z.
- ^ Chihaka abstract
- ^ Mølgaard P, Chihaka A, Lemmich E, et al. (December 2000). "Biodegradability of the molluscicidal saponins of Phytolacca dodecandra". Regul. Toxicol. Pharmacol. 32 (3): 248–55. doi:10.1006/rtph.2000.1390. PMID 11162718. http://linkinghub.elsevier.com/retrieve/pii/S0273-2300(00)91390-4.
- ^ Charnock, Anne (7 August 1980). "Taking Bilharziasis out of the irrigation equation". New Civil Engineer. "Bilharzia caused by poor civil engineering design due to ignorance of cause and prevention"
- ^ The IRG Solution — hierarchical incompetence and how to overcome it. London: Souvenir Press. 1984. p. 88.
- ^ a b c d e The Carter Center. "How is Schistosomiasis Treated?". Archived from the original on 2008-02-25. http://web.archive.org/web/20080225084801/http://www.cartercenter.org/health/schistosomiasis/treatment.html. Retrieved 2008-07-17
- ^ "BILHVAX — A VACCINE AGAINST BILHARZIOSE, A world first!". 20 April 2009. http://www.eurogentec.com/news/104-bilhvax-a-vaccine-against-bilharziose-a-world-first-.html.
- ^ "300.000 morts évitées grâce au vaccin liégeois!". 20 April 2009. http://www.lameuse.be/regions/basse_meuse/2009-04-20/liege-eurogentec-vaccin-extraordinaire-697330.shtml.
- ^ "Eurogentec, la société de biotechnologie située au Sart Tilman, produit et produira le vaccin contre la bilharziose.". 22 April 2009. http://www.lalibre.be/actu/gazette-de-liege/article/497184/un-important-vaccin-produit-a-liege.html.
- ^ Danso-Appiah A, Utzinger J, Liu J, Olliaro P (2008). Danso-Appiah, Anthony. ed. "Drugs for treating urinary schistosomiasis". Cochrane Database Syst Rev (3): CD000053. doi:10.1002/14651858.CD000053.pub2. http://onlinelibrary.wiley.com/o/cochrane/clsysrev/articles/CD000053/frame.html.
- ^ See, for example, Soliman OE, et al. (December 2004). "Evaluation of myrrh (Mirazid) therapy in fascioliasis and intestinal schistosomiasis in children: immunological and parasitological study". J Egypt Soc Parasitol 34 (3): 941–66. PMID 15587320.
- ^ Botros, S; Sayed, H; El-Dusoki, H; Sabry, H; Rabie, I; El-Ghannam, M; Hassanein, M; El-Wahab, YA et al. (February 2005). "Efficacy of mirazid in comparison with praziquantel in Egyptian Schistosoma mansoni-infected school children and households". Am J Trop Med Hyg 72 (2): 119–23. PMID 15741544. http://www.ajtmh.org/cgi/content/full/72/2/119.
- ^ Oliveira, G.; Rodrigues N.B., Romanha, A.J., Bahia, D. (2004). "Genome and Genomics of Schistosomes". Canadian Journal of Zoology 82 (2): 375–90. doi:10.1139/Z03-220. http://www.ingentaconnect.com/content/nrc/cjz/2004/00000082/00000002/art00012.
- ^ Kheir MM, Eltoum IA, Saad AM, Ali MM, Baraka OZ, Homeida MM (February 1999). "Mortality due to schistosomiasis mansoni: a field study in Sudan". Am. J. Trop. Med. Hyg. 60 (2): 307–10. PMID 10072156.
- ^ Strickland GT (May 2006). "Liver disease in Egypt: hepatitis C superseded schistosomiasis as a result of iatrogenic and biological factors". Hepatology 43 (5): 915–22. doi:10.1002/hep.21173. PMID 16628669.
Further reading
- Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Departamento de Vigilância Epidemiológica. 2007. Vigilância e controle de moluscos de importância epidemiológica : diretrizes técnicas : Programa de Vigilância e Controle da Esquistossomose (PCE). Ministério da Saúde, Secretaria de Vigilância em Saúde, Departamento de Vigilância Epidemiológica. 2. ed. Brasília : Editora do Ministério da Saúde. 178 pp. ISBN 978-85-334-1438-9. (in Portuguese) (Surveillance and Control of Mollusks with Epidemiological Importance: technical directives: Schistosomiasis Control and Surveillance Program)
- Crichton-Harris, Ann (2009). Poison in Small Measure: Dr Christopherson and the Cure of Bilharzia. Leiden: Brill.
External links
Diseases of poverty Diseases of poverty Neglected diseases Cholera · Chagas disease · African Sleeping Sickness · Schistosomiasis · Guinea worm · River blindness · LeishmaniasisMiscellaneous Categories:- Waterborne diseases
- Helminthiases
- Zoonoses
- Tropical diseases
- Hepatology
- Neglected diseases
Wikimedia Foundation. 2010.




