- Oxamniquine
-
Oxamniquine 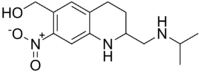
Systematic (IUPAC) name (RS)-1,2,3,4-Tetrahydro-2-isopropylaminomethyl-7-nitro-6-quinolylmethanol Clinical data AHFS/Drugs.com Micromedex Detailed Consumer Information Pregnancy cat. It is not known whether it will harm an unborn baby (FDA Pregnancy Category C) Legal status Not commercially available in the United States Routes oral Pharmacokinetic data Bioavailability Readily absorbed after oral doses Metabolism hepatic Half-life 1 to 2.5h Excretion mainly in urine Identifiers CAS number 21738-42-1 ATC code P02BA02 QP52AA02 PubChem CID 4612 DrugBank APRD01150 ChemSpider 4451 
UNII 0O977R722D 
KEGG D00460 
ChEMBL CHEMBL847 
Chemical data Formula C14H21N3O3 Mol. mass 279.3 SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Oxamniquine is an anthelmintic with schistosomicidal activity against Schistosoma mansoni, but not against other Schistosoma spp. Oxamniquine is a potent single-dose agent for treatment of S. mansoni infection in man, and it causes worms to shift from the mesenteric veins to the liver, where the male worms are retained; the female worms return to the mesentery, but can no longer release eggs.[1]
Contents
History
Oxamniquine was first described by Kaye and Woolhouse in 1972 as a metabolite of the compound UK 3883 (2-isopropylaminomethyl-6-methyl-7-nitro-1,2,3,4-tetrahydroquinoline). Initially, it was prepared by microbiological hydroxylation in the presence of the fungus Aspergillus sclerotiorum. In 1979, Pfizer at Sandwich was presented with the Queen's Award for Technological Achievement in recognition of the outstanding contribution made to tropical medicine by MANSIL (oxamniquine).
Pharmacokinetics
Peak plasma concentrations are achieved one to three hours after a dose, and the plasma half-life is 1.0 to 2.5 hours.
It is extensively metabolised to inactive metabolites, principally the 6-carboxy derivative, which are excreted in the urine. About 70% of a dose of oxamniquine is excreted as the 6-carboxy metabolite within 12 hours of a dose; traces of the 2-carboxy metabolite have also been detected in the urine.
Mode of action
Oxamniquine is a semisynthetic tetrahydroquinoline and possibly acts by DNA binding, resulting in contraction and paralysis of the worms and eventual detachment from terminal venules in the mesentry, and death. Its biochemical mechanisms are hypothesized to be related to an anticholinergic effect, which increases the parasite’s motility, as well as to synthesis inhibition of nucleic acids. Oxamniquine acts mainly on male worms, but also induces small changes on a small proportion of females. Like praziquantel, it promotes more severe damage of the dorsal tegument than of the ventral surface. The drug causes the male worms to shift from the mesenteric circulation to the liver, where the cellular host response causes its final elimination. The changes caused in the females are reversible and are due primarily to the discontinued male stimulation rather than the direct effect of oxamniquine.
Uses
Oxamniquine is used for treatment of schistosomiasis. According to one systematic review, it is equally effective as praziquantel for treating S. mansoni infections.[citation needed]
Contraindications and precautions
Oxamniquine should not be taken during pregnancy.[citation needed]
Side effects
It is generally well tolerated following oral doses. Dizziness with or without drowsiness occurs in at least a third of patients, beginning up to three hours after a dose, and usually lasts for up to six hours. Headache and gastrointestinal effects, such as nausea, vomiting, and diarrhoea, are also common.
Allergic-type reactions, including urticaria, pruritic skin rashes, and fever, may occur. Liver enzyme values have been raised transiently in some patients. Epileptiform convulsions have been reported, especially in patients with a history of convulsive disorders. Hallucinations and excitement have occurred rarely.
A reddish discoloration of urine, probably due to a metabolite of oxamniquine, has been reported.
Dosage
Oral, 15 mg per kg of body weight two times a day for one day.
Brand names
- Vansil; (Pfizer) 250 mg capsules, syrup 250 mg/5 mL
- Mansil; 250 mg Tablets
References
- ^ Martidale, The Extra Pharmacopoeia, 31st ed, p121
- AHFS Database
External links
Antiparasitics – Anthelmintics (P02) Antiplatyhelmintic agents Antitrematodals
(schistosomicides)Binds tubulinOther/unknownquinoline (Praziquantel#, Oxamniquine#) • phenol (Bithionol) • thiazole (Niridazole) • arylsulfonate (Stibophen)Anticestodals
(taeniacides)Binds tubulinOther/unknownsalicylanilide (Niclosamide)# • aminoacridine (Quinacrine) • butyrophenone (Desaspidin) • chlorophenol (Dichlorophen)Antinematodal agents
(including
macrofilaricides)Binds tubulinbenzimidazole (Mebendazole#, Albendazole#, Thiabendazole, Fenbendazole, Ciclobendazole, Flubendazole)Other/unknownpiperazine (Piperazine • Diethylcarbamazine#) • thiazole (Levamisole#) • quinolinium (Pyrvinium) • benzylammonium (Bephenium) • naphthalenesulfonate (Suramin#) • TribendimidineM: IFT
helm,arth (acar)
helm, arth (lice), zoon
helm, arth
Categories:- Antiparasitic agents
- Quinolines
- Alcohols
- Nitro compounds
- Amines
Wikimedia Foundation. 2010.
