- Levamisole
-
Levamisole 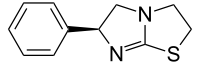
Systematic (IUPAC) name (S)-6-Phenyl-2,3,5,6-tetrahydroimidazo[2,1-b][1,3]thiazole Clinical data AHFS/Drugs.com Micromedex Detailed Consumer Information MedlinePlus a697011 Pregnancy cat. ? Legal status ? Routes Oral Pharmacokinetic data Metabolism Hepatic Half-life 4.4-5.6 hours (biphasic) Identifiers CAS number 14769-73-4 
ATC code P02CE01 QP52AE01 PubChem CID 26879 DrugBank APRD01067 ChemSpider 25037 
UNII 2880D3468G 
KEGG D08114 
ChEMBL CHEMBL1454 
Chemical data Formula C11H12N2S Mol. mass 204.292 g/mol SMILES eMolecules & PubChem Physical data Density 1.31 g/cm³ Melt. point 230 °C (446 °F)  (what is this?) (verify)
(what is this?) (verify)Levamisole (marketed as the hydrochloride salt under the trade name Ergamisol) is an anthelminthic and immunomodulator belonging to a class of synthetic imidazothiazole derivatives. It was discovered at Janssen Pharmaceutica in 1966. Levamisole has been used in humans to treat parasitic worm infections, and has been studied in combination with other forms of chemotherapy for colon cancer, melanoma, and head and neck cancer. The drug was withdrawn from the U.S. and Canadian markets in 2000 and 2003 respectively, due to the risk of serious side effects and the availability of more effective replacement medications.[1][2] The key side toxic effect of the drug is agranulocytosis, a severe depletion of white blood cells that leaves patients vulnerable to infection.
Currently, levamisole remains in veterinary use as a dewormer for livestock. The medication has also been increasingly used as an adulterant in cocaine sold in the U.S. and Canada, resulting in serious side effects.[3][4]
Contents
Medical uses
Levamisole was originally used as an anthelminthic to treat worm infestations in both humans and animals. Most current commercial preparations are intended for veterinary use as a dewormer in cattle, pigs, and sheep. However, levamisole has also gained prominence among aquarists as an effective treatment for Camallanus roundworm infestations in freshwater tropical fish.[5]
Levamisole has been tested in combination with fluorouracil to treat colon cancer. There is no good evidence from clinical trials that its addition to fluorouracil therapy benefits patients with colon cancer, and it is no longer used for this. Levamisole is also used infrequently to treat melanoma and head and neck cancer. It is unclear from its mechanism of action why it would have an effect in treating colon cancer, although it has been shown to have "immune-stimulating" properties in some situations.
A 1984 study on complicated influenza found levamisole to be an effective inducer of interferon and recommended its use in combination therapy for influenza.[6] It is also used routinely in India to treat steroid-dependent childhood nephrotic syndrome cases, based on literature published in a few U.K.-based studies.[7] Levamisole has been used to treat a variety of dermatologic conditions, including skin infections, leprosy, warts, lichen planus, and aphthous ulcers.[8]
Laboratory use
Levamisole reversibly and non-competitively inhibits most isoforms of alkaline phosphatase (e.g. human liver, bone, kidney, and spleen) except the intestinal and placental isoform.[9] It is thus used as an inhibitor along with substrate to reduce background alkaline phosphatase activity in biomedical assays involving detection signal amplification by intestinal alkaline phosphatase, for example in in situ hybridization or Western blot protocols.
In addition, it is used to immobilize the nematode C. elegans on glass slides for imaging.
Illicit use
Levamisole has increasingly been used as a cutting agent in cocaine sold in the U.S. and Canada. In 2008–2009, levamisole was found in 69% of cocaine samples seized by the U.S. Drug Enforcement Administration (DEA).[3] By April 2011, the DEA reported that the adulterant was found in 82% of seizures.[10]
Levamisole adds bulk and weight to powdered cocaine (whereas other adulterants produce smaller "rocks" of cocaine) and makes the drug appear more pure.[11] In a series of investigative articles for The Stranger, Brendan Kiley details other rationales for levamisole's rise as an adulterant: possible stimulant effects, a similar appearance to cocaine, and an ability to pass street purity tests.[12]
Levamisole suppresses the production of white blood cells, resulting in neutropenia and agranulocytosis. With the increasing use of levamisole as an adulterant, a number of these complications have been reported among cocaine users.[3][13][14] Levamisole has also been linked to a risk of vasculitis,[15] and 2 cases of vasculitic skin necrosis have been reported in users of cocaine adulterated with levamisole.[16]
Levamisole-tainted cocaine was linked to several high-profile deaths. Toxicology reports showed that levamisole, along with cocaine, was present in DJ AM's body at the time of his death.[17][dead link] Andrew Koppel, son of newsman Ted Koppel, was also found with Levamisole in his body after his death was ruled a drug overdose.[18]
Detection in body fluids
Levamisole may be quantified in blood, plasma or urine as a diagnostic tool in clinical poisoning situations or to aid in the medicolegal investigation of suspicious deaths involving adulterated street drugs. About 3% of an oral dose is eliminated unchanged in the 24 hour urine of humans. A postmortem blood levamisole concentration of 2.2 mg/L was present in a woman who died of a cocaine overdose.[19][20]
References
- ^ http://www.ncbi.nlm.nih.gov/pubmed/20668440
- ^ "Products Discontinued from the Market Since Publication of the 2000 CPS". Canadian Pharmacists Association. http://www.pharmacists.ca/content/hcp/tools/drugnews/discontinued.htm. Retrieved 2009-08-13.
- ^ a b c Centers for Disease Control and Prevention (CDC) (December 2009). "Agranulocytosis associated with cocaine use - four States, March 2008-November 2009". MMWR Morb. Mortal. Wkly. Rep. 58 (49): 1381–5. PMID 20019655. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5849a3.htm.
- ^ http://www.ncbi.nlm.nih.gov/pubmed/20668440
- ^ Sanford, Shari (2007). "Levamisole Hydrochloride: Its application and usage in freshwater aquariums". Loaches Online. http://www.loaches.com/disease-treatment/levamisole-hydrochloride-1. Retrieved 2009-02-27.
- ^ Grishchenko SV, Lavrukhina LA, Ketiladze ES, Krylov VF, Ershov FI (1984). "[Results of combined therapy using levamisole for patients with influenza complicated by pneumonia]" (in Russian). Vopr. Virusol. 29 (2): 175–9. PMID 6203228.
- ^ "Levamisole for corticosteroid-dependent nephrotic syndrome in childhood. British Association for Paediatric Nephrology". Lancet 337 (8757): 1555–7. 1991. PMID 1675705.
- ^ Scheinfeld N, Rosenberg JD, Weinberg JM (2004). "Levamisole in dermatology : a review". Am J Clin Dermatol 5 (2): 97–104. doi:10.2165/00128071-200405020-00004. PMID 15109274.
- ^ Van Belle H (1976). "Alkaline phosphatase. I. Kinetics and inhibition by levamisole of purified isoenzymes from humans". Clin. Chem. 22 (7): 972–6. PMID 6169.
- ^ Moisse, Katie (2011-06-23). "Cocaine Laced With Veterinary Drug Levamisole Eats Away at Flesh". ABC News. http://abcnews.go.com/Health/Wellness/flesh-eating-cocaine-laced-veterinary-drug-levamisole/story?id=13902353. Retrieved 2011-06-23.
- ^ Doheny, Kathleen (Jun 1 2010). "Contaminated Cocaine Can Cause Flesh to Rot". Yahoo!. http://news.yahoo.com/s/hsn/contaminatedcocainecancausefleshtorot;_ylt=Arpc.e2vZk4K0VyhIadneKes0NUE;_ylu=X3oDMTRhMzAybmR2BGFzc2V0A2hzbi8yMDEwMDYwMS9jb250YW1pbmF0ZWRjb2NhaW5lY2FuY2F1c2VmbGVzaHRvcm90BGNjb2RlA21vc3Rwb3B1bGFyBGNwb3MDOARwb3MDNQRwdANob21lX2Nva2UEc2VjA3luX2hlYWRsaW5lX2xpc3QEc2xrA2NvbnRhbWluYXRlZA--. Retrieved 8 June 2010.[dead link]
- ^ Kiley, Brendan (August 17, 2010). "The Mystery of the Tainted Cocaine". The Stranger. http://www.thestranger.com/seattle/the-mystery-of-the-tainted-cocaine/Content?oid=4683741. Retrieved December 21, 2010.
- ^ Nancy Y Zhu, Donald F. LeGatt, A Robert Turner (February 2009). "Agranulocytosis After Consumption of Cocaine Adulterated With Levamisole". Annals of Internal Medicine 150 (4): 287–289. doi:10.1059/0003-4819-150-4-200902170-00102. PMID 19153405. http://www.annals.org/cgi/content/short/150/4/287. Retrieved 2009-10-07.
- ^ Kinzie, Erik (April 2009). "Levamisole Found in Patients Using Cocaine". Annals of Emergency Medicine 53 (4): 546–7. doi:10.1016/j.annemergmed.2008.10.017. PMID 19303517. http://www.mdconsult.com/das/article/body/154945316-2/jorg=journal&source=&sp=21877276&sid=0/N/691072/1.html?issn=01960644. Retrieved 2009-08-18.
- ^ Menni S, Pistritto G, Gianotti R, Ghio L, Edefonti A (1997). "Ear lobe bilateral necrosis by levamisole-induced occlusive vasculitis in a pediatric patient". Pediatr Dermatol 14 (6): 477–9. doi:10.1111/j.1525-1470.1997.tb00695.x. PMID 9436850.
- ^ Bradford M, Rosenberg B, Moreno J, Dumyati G (June 2010). "Bilateral necrosis of earlobes and cheeks: another complication of cocaine contaminated with levamisole". Ann. Intern. Med. 152 (11): 758–9. doi:10.1059/0003-4819-152-11-201006010-00026. PMID 20513844.
- ^ The San Francisco Chronicle. http://www.sfgate.com/cgi-bin/article.cgi?f=/n/a/2009/09/29/national/a075802D66.DTL&tsp=1.[dead link]
- ^ Parascandola, Rocco (2010-06-18). "Ted Koppel's son, Andrew Koppel, overdosed on cocktail containing booze, heroin, cocaine and Valium". Daily News (New York). http://www.nydailynews.com/ny_local/2010/06/19/2010-06-19_koppel_son_cocktail_drug_death_an_accident.html.
- ^ Vandamme TF, Demoustier M, Rollmann B. Quantitation of levamisole in plasma using high performance liquid chromatography. Eur. J. Drug Metab. Pharmacokinet. 20: 145-149, 1995.
- ^ R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 9th edition, Biomedical Publications, Seal Beach, CA, 2011, pp.901-902. http://www.biomedicalpublications.com/levamisole.pdf.
Antiparasitics – Anthelmintics (P02) Antiplatyhelmintic agents Antitrematodals
(schistosomicides)Binds tubulinOther/unknownquinoline (Praziquantel#, Oxamniquine#) • phenol (Bithionol) • thiazole (Niridazole) • arylsulfonate (Stibophen)Anticestodals
(taeniacides)Binds tubulinOther/unknownsalicylanilide (Niclosamide)# • aminoacridine (Quinacrine) • butyrophenone (Desaspidin) • chlorophenol (Dichlorophen)Antinematodal agents
(including
macrofilaricides)Binds tubulinbenzimidazole (Mebendazole#, Albendazole#, Thiabendazole, Fenbendazole, Ciclobendazole, Flubendazole)Other/unknownpiperazine (Piperazine • Diethylcarbamazine#) • thiazole (Levamisole#) • quinolinium (Pyrvinium) • benzylammonium (Bephenium) • naphthalenesulfonate (Suramin#) • TribendimidineM: IFT
helm,arth (acar)
helm, arth (lice), zoon
helm, arth
Categories:- Anthelmintics
- Janssen Pharmaceutica
Wikimedia Foundation. 2010.
