- Decitabine
-
Decitabine 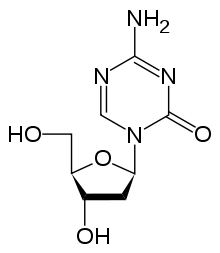
Systematic (IUPAC) name 4-amino-1-(2-deoxy-b-D-erythro-pentofuranosyl)-
1,3,5-triazin-2(1H)-oneClinical data AHFS/Drugs.com monograph MedlinePlus a608009 Pregnancy cat. D Legal status ? Routes Intravenous Pharmacokinetic data Protein binding <1% Half-life 30 minutes Identifiers CAS number 2353-33-5 ATC code L01BC08 PubChem CID 451668 DrugBank DB01262 ChemSpider 397844 
UNII 776B62CQ27 
KEGG D03665 
ChEBI CHEBI:50131 
ChEMBL CHEMBL66115 
Chemical data Formula C8H12N4O4 Mol. mass 228.206 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Decitabine (trade name Dacogen), or 5-aza-2'-deoxycytidine, is a cytidine analog.
Contents
Mechanism
It is a hypomethylating agent.[1][2] It hypomethylates DNA by inhibiting DNA methyltransferase.
It functions in a similar manner to azacitidine, although decitabine can only be incorporated into DNA strands while azacitidine can be incorporated into both DNA and RNA chains.
Clinical uses
Decitabine is indicated for the treatment of myelodysplastic syndromes (MDS) including previously treated and untreated, de novo and secondary MDS of all French-American-British subtypes (refractory anemia, refractory anemia with ringed sideroblasts, refractory anemia with excess blasts, refractory anemia with excess blasts in transformation, and chronic myelomonocytic leukemia) and Intermediate-1, Intermediate-2, and High-Risk International Prognostic Scoring System groups.
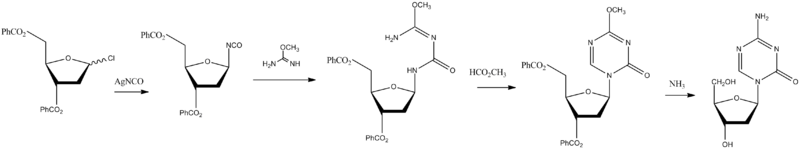
Chemical Synth
Piml, J.; Sorm, F.; Coll. Czech. Chem. Commun. 1964, 29, 2576.
Further reading
- Moon C, Kim SH (June 2009). "Use of epigenetic modification to induce FOXP3 expression in naïve T cells". Transplant Proc. 41 (5): 1848–54. doi:10.1016/j.transproceed.2009.02.101. PMID 19545742.
References
- ^ Kantarjian H, Issa JP, Rosenfeld CS, et al. (April 2006). "Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study". Cancer 106 (8): 1794–803. doi:10.1002/cncr.21792. PMID 16532500.
- ^ Kantarjian HM, O'Brien S, Cortes J, et al. (August 2003). "Results of decitabine (5-aza-2'deoxycytidine) therapy in 130 patients with chronic myelogenous leukemia". Cancer 98 (3): 522–8. doi:10.1002/cncr.11543. PMID 12879469.
External links

This antineoplastic or immunomodulatory drug article is a stub. You can help Wikipedia by expanding it.