- Omacetaxine mepesuccinate
-
Omacetaxine mepesuccinate 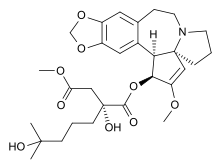
Systematic (IUPAC) name 1-((1S,3aR,14bS)-2-Methoxy-1,5,6,8,9,14b-hexahydro-4H-cyclopenta(a)(1,3)dioxolo(4,5-h)pyrrolo(2,1-b)(3)benzazepin-1-yl) 4-methyl (2R)-2-hydroxy-2-(4-hydroxy-4-methylpentyl)butanedioate Clinical data Pregnancy cat. ? Legal status ? Routes Subcutaneous, intravenous infusion Identifiers CAS number 26833-87-4 ATC code L01XX40 PubChem CID 285033 UNII 6FG8041S5B 
Chemical data Formula C29H39NO9 Mol. mass 545.62 g/mol SMILES eMolecules & PubChem - InChI=1S/C29H39NO9/c1-27(2,33)8-5-10-29(34,16-23(31)36-4)26(32)39-25-22(35-3)15-28-9-6-11-30(28)12-7-18-13-20-21(38-17-37-20)14-19(18)24(25)28/h13-15,24-25,33-34H,5-12,16-17H2,1-4H3/t24-,25-,28+,29-/m1/s1
Key:HYFHYPWGAURHIV-JFIAXGOJSA-N
Omacetaxine mepesuccinate (INN, or homoharringtonine, trade name Omapro) is an alkaloid from Cephalotaxus harringtonia that is investigated for potential use as a drug against hematological cancers. It is being developed by ChemGenex and is on fast track approval schedule in the United States. Omacetaxine has been granted orphan drug status in the U.S. and in Europe.[1]
Mechanism of action
Omacetaxine induces apoptosis by inhibition of protein synthesis, particularly Mcl-1. It has a different point of action than tyrosine kinase inhibitors like imatinib, and has potential therapeutic advantages for patients who have developed resistance to tyrosine kinase inhibitor therapy.[2]
Studies
In vitro and animal model trials showed that omacetaxine has potential to treat resistant leukemia mainly chronic myelogenous leukemia (CML) and acute lymphoblastic leukemia (ALL).[3]
In June 2009, results of a long-term open label Phase II study were published, which investigated the use of omacetaxine infusions in CML patients. After twelve months of treatment, about one third of patients showed a cytogenetic response.[4] A study in patients who had failed imatinib and who had the drug resistant T315I mutation achieved cytogenetic response in 28% of patients and hematological response in 80% of patients, according to preliminary data.[5]
Phase I studies including a small number of patients have shown benefit in treating myelodysplastic syndrome (MDS, 25 patients)[6] and acute myelogenous leukemia (AML, 76 patients).[7] Patients with solid tumors did not benefit from omacetaxine.[8]
References
- ^ "NDA Submitted for Omapro". Drugs.com. September 9, 2009. http://www.drugs.com/nda/omapro_090909.html.
- ^ "ChemGenex Investigators Report Activity of omacetaxine in imatinib-resistant chronic myeloid leukemia Patients with the T315I Mutation". December 10, 2007. http://www.chemgenex.com/2007/12/chemgenex-investigators-report-activity-of-omacetaxine-in-imatinib-resistant-chronic-myeloid-leukemia-patients-with-the-t315i-mutation/.
- ^ Will Boggs (April 14, 2009). "Omacetaxine has potential to treat resistant leukemia". Reuters. http://www.oncolink.org/resources/article.cfm?c=3&s=8&ss=23&Year=2009&Month=4&id=16157.
- ^ Li, YF; Deng, ZK; Xuan, HB; Zhu, JB; Ding, BH; Liu, XN; Chen, BA (2009). "Prolonged chronic phase in chronic myelogenous leukemia after homoharringtonine therapy". Chinese medical journal 122 (12): 1413–7. PMID 19567163.
- ^ Quintás-Cardama, A.; Kantarjian, H.; Cortes, J. (2009). "Homoharringtonine, omacetaxine mepesuccinate, and chronic myeloid leukemia circa 2009". Cancer 115 (23): 5382. doi:10.1002/cncr.24601. PMID 19739234.
- ^ Wu, L.; Li, X.; Su, J.; Chang, C.; He, Q.; Zhang, X.; Xu, L.; Song, L. et al. (2009). "Effect of low-dose cytarabine, homoharringtonine and granulocyte colony-stimulating factor priming regimen on patients with advanced myelodysplastic syndrome or acute myeloid leukemia transformed from myelodysplastic syndrome". Leukemia & Lymphoma 50: 1461. doi:10.1080/10428190903096719.
- ^ Gu, L. F.; Zhang, W. G.; Wang, F. X.; Cao, X. M.; Chen, Y. X.; He, A. L.; Liu, J.; Ma, X. R. (2010). "Low dose of homoharringtonine and cytarabine combined with granulocyte colony-stimulating factor priming on the outcome of relapsed or refractory acute myeloid leukemia". Journal of Cancer Research and Clinical Oncology. doi:10.1007/s00432-010-0947-z.
- ^ Kantarjian, H. M.; Talpaz, M.; Santini, V.; Murgo, A.; Cheson, B.; O'Brien, S. M. (2001). "Homoharringtonine". Cancer 92 (6): 1591. doi:10.1002/1097-0142(20010915)92:6<1591::AID-CNCR1485>3.0.CO;2-U. PMID 11745238.

This antineoplastic or immunomodulatory drug article is a stub. You can help Wikipedia by expanding it. - InChI=1S/C29H39NO9/c1-27(2,33)8-5-10-29(34,16-23(31)36-4)26(32)39-25-22(35-3)15-28-9-6-11-30(28)12-7-18-13-20-21(38-17-37-20)14-19(18)24(25)28/h13-15,24-25,33-34H,5-12,16-17H2,1-4H3/t24-,25-,28+,29-/m1/s1
