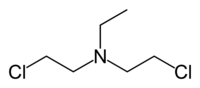
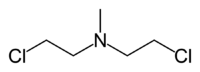
- Nitrogen mustard
-
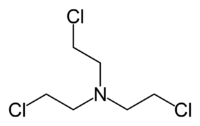 HN3 (tris(2-chloroethyl)amine)
HN3 (tris(2-chloroethyl)amine)
The nitrogen mustards are cytotoxic chemotherapy agents similar to mustard gas. Although their common use is medicinal, in principle these compounds can also be deployed as chemical warfare agents. Nitrogen mustards are nonspecific DNA alkylating agents. Nitrogen mustard gas was stockpiled by several nations during the Second World War, but it was never used in combat. As with all types of mustard gas, nitrogen mustards are powerful and persistent blister agents and the main examples (HN1, HN2, HN3, see below) are therefore classified as Schedule 1 substances within the Chemical Weapons Convention. Production and use is therefore strongly restricted.
During WWII nitrogen mustards were studied at Yale University and classified human clinical trials of nitrogen mustards for the treatment of lymphoma started in December 1942.[1] Also during WWII, an incident during the air raid on Bari, Italy led to the release of mustard gas that affected several hundred soldiers and civilians. Medical examination of the survivors showed a decreased number of lymphocytes.[2] After WWII was over, the Bari incident and the Yale group's studies eventually converged prompting a search for other similar compounds. Due to its use in previous studies, the nitrogen mustard known as "HN2" became the first chemotherapy drug mustine.
Examples
The original nitrogen mustard drug, mustine (HN2), is no longer commonly in use. Other nitrogen mustards developed as treatments include cyclophosphamide, chlorambucil, uramustine, ifosfamide, melphalan and bendamustine. Bendamustine has recently reemerged as a viable chemotheraputic treatment.[3]
Nitrogen mustards that can be used for chemical warfare purposes are tightly regulated. Their weapon designations are:
- HN1: Bis(2-chloroethyl)ethylamine
- HN2: Bis(2-chloroethyl)methylamine
- HN3: Tris(2-chloroethyl)amine
Mechanism of action
Nitrogen mustards (NMs) form cyclic aminium ions (aziridinium rings) by intramolecular displacement of the chloride by the amine nitrogen. This azidirium group then alkylates DNA by attacking the N-7 nucleophilic center on the guanine base. A second attack after the displacement of the second chlorine forms the second alkylation step that results in the formation of interstrand cross-links (ICLs) as it was shown in the early 1960s. At that time it was proposed that the ICLs were formed between N-7 atom of guanine residue in a 5’-d(GC) sequence.[4][5] These kind of lesion are highly cytotoxic, since they block fundamental metabolic processes such as replication and transcription.
Later was it clearly demonstrated that NMs form a 1,3 ICL in the 5’-d(GNC) sequence.[6][7][8]
The strong cytotoxic effect caused by the formation of ICLs is what makes NMs an effective chemotherapeutic agent. Other compounds used in cancer chemotherapy that have the ability to form ICLs are cisplatin, mitomycin C, carmustine, psoralen.
References
- ^ Gilman A (May 1963). "The initial clinical trial of nitrogen mustard". Am. J. Surg. 105 (5): 574–8. doi:10.1016/0002-9610(63)90232-0. PMID 13947966.
- ^ Hirsch J (September 2006). "An anniversary for cancer chemotherapy". JAMA 296 (12): 1518–20. doi:10.1001/jama.296.12.1518. PMID 17003400.
- ^ Cheson BD, Rummel MJ (March 2009). "Bendamustine: rebirth of an old drug". J. Clin. Oncol. 27 (9): 1492–501. doi:10.1200/JCO.2008.18.7252. PMID 19224851. http://www.jco.org/cgi/pmidlookup?view=long&pmid=19224851.
- ^ Geiduschek EP (July 1961). ""Reversible" DNA". Proc. Natl. Acad. Sci. U.S.A. 47 (7): 950–5. doi:10.1073/pnas.47.7.950. PMC 221307. PMID 13704192. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=221307.
- ^ Brookes P, Lawley PD (September 1961). "The reaction of mono- and di-functional alkylating agents with nucleic acids". Biochem. J. 80 (3): 496–503. PMC 1243259. PMID 16748923. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1243259.
- ^ Rink SM, Solomon MS, Taylor MJ, Rajur SB, McLaughlin LW, Hopkins PB (1993). "Covalent structure of a nitrogen mustard-induced DNA interstrand cross-link: An N7-to-N7 linkage of deoxyguanosine residues at the duplex sequence 5'-d(GNC)". Journal of the American Chemical Society 115 (7): 2551–7. doi:10.1021/ja00060a001.
- ^ Dong Q, Barsky D, Colvin ME, et al. (December 1995). "A structural basis for a phosphoramide mustard-induced DNA interstrand cross-link at 5'-d(GAC)". Proc. Natl. Acad. Sci. U.S.A. 92 (26): 12170–4. doi:10.1073/pnas.92.26.12170. PMC 40318. PMID 8618865. http://www.pnas.org/cgi/pmidlookup?view=long&pmid=8618865.
- ^ Bauer GB, Povirk LF (March 1997). "Specificity and kinetics of interstrand and intrastrand bifunctional alkylation by nitrogen mustards at a G-G-C sequence". Nucleic Acids Res. 25 (6): 1211–8. doi:10.1093/nar/25.6.1211. PMC 146567. PMID 9092631. http://nar.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=9092631.
Blood Blister Ethyldichloroarsine (ED) · Methyldichloroarsine (MD) · Phenyldichloroarsine (PD) · Lewisite (L) · Sulfur mustard (HD · H · HT · HL · HQ) · Nitrogen mustard (HN1 · HN2 · HN3)
Nerve Pulmonary Incapacitating Riot control Categories:- Alkylating antineoplastic agents
- Nitrogen
- Blister agents
- IARC Group 2A carcinogens
- Nitrogen mustards
Wikimedia Foundation. 2010.