- Melphalan
-
Melphalan 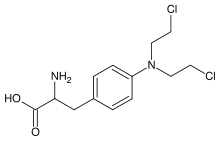
Systematic (IUPAC) name 4-[bis(chloroethyl)amino]phenylalanine Clinical data Trade names Alkeran AHFS/Drugs.com monograph MedlinePlus a682220 Pregnancy cat. ? Legal status ℞ Prescription only Routes Oral, intravenous Pharmacokinetic data Bioavailability 25% to 89% Metabolism hydrolysis Half-life 1.5 ± 0.8 hours Excretion Renal, significantly metabolised Identifiers CAS number 148-82-3 
ATC code L01AA03 PubChem CID 4053 DrugBank APRD00118 ChemSpider 405297 
UNII Q41OR9510P 
KEGG D00369 
ChEBI CHEBI:28876 
ChEMBL CHEMBL852 
Synonyms 2-amino-3-[4-[bis(2-chloroethyl)amino]phenyl]-propanoic acid Chemical data Formula C13H18Cl2N2O2 Mol. mass 305.2 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Melphalan hydrochloride (trade name Alkeran) is a chemotherapy drug belonging to the class of nitrogen mustard alkylating agents.
An alkylating agent adds an alkyl group (CnH2n+1) to DNA. It attaches the alkyl group to the guanine base of DNA, at the number 7 nitrogen atom of the imidazole ring.
Otherwise known as L-Phenylalanine Mustard, or L-PAM, melphalan is a phenylalanine derivative of mechlorethamine.
Contents
Uses
It is used to treat multiple myeloma[1] and ovarian cancer, and occasionally malignant melanoma.
The agent was first investigated as a possible drug for use in melanoma. It was not found to be effective, but has been found to be effective in the treatment of myeloma.
Administration
Oral or intravenous; dosing varies by purpose and route of administration as well as patient weight.
Melphalan Prescribing Information: Alkeran[2]
Melphalan Patient Information: MedlinePlus[3]
Melphalan Material Safety Data Sheet (MSDS): Sequoia Research Products[4]
Side effects
Common side effects include:
- Nausea and vomiting, and oral ulceration.
- Bone marrow suppression, including
- Decreased white blood cell count causing increased risk of infection
- Decreased platelet count causing increased risk of bleeding
Less common side effects include:
- Severe allergic reactions
- Pulmonary fibrosis (scarring of lung tissue) including fatal outcomes (usually only with prolonged use)
- Hair loss
- Interstitial pneumonitis
- Rash
- Itching
- Irreversible bone marrow failure due to melphalan not being withdrawn early enough.
- Cardiac arrest.
References
- ^ Facon T, Mary JY, Hulin C, et al. (October 2007). "Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): a randomised trial". Lancet 370 (9594): 1209–18. doi:10.1016/S0140-6736(07)61537-2. PMID 17920916. http://linkinghub.elsevier.com/retrieve/pii/S0140-6736(07)61537-2.
- ^ celgene.com
- ^ nlm.nih.gov
- ^ seqchem.com

This antineoplastic or immunomodulatory drug article is a stub. You can help Wikipedia by expanding it.
