- Plicamycin
-
Plicamycin 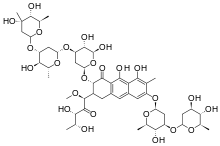
Systematic (IUPAC) name (1S)-5-deoxy-1-C-((2S,3S)-7-{[2,6-dideoxy-3-O-(2,6-dideoxy-β-D-arabino-hexopyranosyl)-β-D-arabino-hexopyranosyl]oxy}-3-{[2,6-dideoxy-3-C-methyl-β-D-ribo-hexopyranosyl-(1→3)-2,6-dideoxy-β-D-arabino-hexopyranosyl-(1→3)-2,6-dideoxy-β-D-arabino-hexopyranosyl]oxy}-5,10-dihydroxy-6-methyl-4-oxo-1,2,3,4-tetrahydroanthracen-2-yl)-1-O-methyl-D-xylulose Clinical data AHFS/Drugs.com Micromedex Detailed Consumer Information Pregnancy cat. ? Legal status ? Routes Intravenous Identifiers CAS number 18378-89-7 
ATC code L01DC02 PubChem CID 5284610 DrugBank DB06810 ChemSpider 4447655 
UNII NIJ123W41V 
KEGG D00468 
ChEMBL CHEMBL509846 
Synonyms Aureolic acid; Mithracin; Antibiotic LA 7017; Mithramycin A; Mitramycin; Plicatomycin Chemical data Formula C52H76O24 Mol. mass 1085.15 g/mol - InChI=1S/C52H76O24/c1-18-29(72-34-14-30(43(58)21(4)68-34)73-33-13-28(54)42(57)20(3)67-33)12-26-10-25-11-27(49(66-9)48(63)41(56)19(2)53)50(47(62)39(25)46(61)38(26)40(18)55)76-36-16-31(44(59)23(6)70-36)74-35-15-32(45(60)22(5)69-35)75-37-17-52(8,65)51(64)24(7)71-37/h10,12,19-24,27-28,30-37,41-45,49-51,53-61,64-65H,11,13-17H2,1-9H3/t19-,20-,21-,22-,23-,24-,27?,28-,30-,31-,32-,33+,34+,35+,36+,37+,41+,42-,43+,44-,45-,49+,50+,51-,52+/m1/s1

Key:CFCUWKMKBJTWLW-GWRQQDNDSA-N
 (what is this?) (verify)
(what is this?) (verify)Plicamycin (INN, also known as mithramycin; trade name Mithracin) is an antineoplastic antibiotic produced by Streptomyces plicatus. It is an RNA synthesis inhibitor.[1] The manufacturer discontinued production in 2000.
Uses
Plicamycin has been used in the treatment of testicular cancer,[2][3] Paget's disease of bone,[4][5] and, rarely, the management of hypercalcemia.
Plicamycin has been tested in chronic myeloid leukemia.[6]
Plicamycin is currently used in multiple areas of research, including cancer cell apoptosis[7] and as a metastasis inhibitor.[8]
One elucidated pathway shows it interacts by cross-binding chromatin GC-rich promoter motifs, thereby inhibiting gene transcription.[9]
References
- ^ "Mithramycin A". Fermentek. http://www.fermentek.co.il/mithramycin_A.htm.
- ^ Kennedy BJ, Torkelson JL (May 1995). "Long-term follow-up of stage III testicular carcinoma treated with mithramycin (plicamycin)". Med. Pediatr. Oncol. 24 (5): 327–8. doi:10.1002/mpo.2950240511. PMID 7700186.
- ^ Brown, John H.; Kennedy, B. J. (1965). "Mithramycin in the Treatment of Disseminated Testicular Neoplasms". New England Journal of Medicine 272 (3): 111–8. doi:10.1056/NEJM196501212720301. PMID 14224214.
- ^ Hall, T; Schaeublin, M; Chambers, TJ (1993). "The Majority of Osteoclasts Require mRNA and Protein Synthesis for Bone Resorption in Vitro". Biochemical and Biophysical Research Communications 195 (3): 1245–53. doi:10.1006/bbrc.1993.2178. PMID 8216256.
- ^ Remsing, Lily L.; Bahadori, Hamid R.; Carbone, Giuseppina M.; McGuffie, Eileen M.; Catapano, Carlo V.; Rohr, Jürgen (2003). "Inhibition of c-src Transcription by Mithramycin: Structure−Activity Relationships of Biosynthetically Produced Mithramycin Analogues Using the c-src Promoter as Target". Biochemistry 42 (27): 8313–24. doi:10.1021/bi034091z. PMID 12846580.
- ^ Dutcher JP, Coletti D, Paietta E, Wiernik PH (May 1997). "A pilot study of alpha-interferon and plicamycin for accelerated phase of chronic myeloid leukemia". Leuk. Res. 21 (5): 375–80. doi:10.1016/S0145-2126(96)00108-7. PMID 9225062. http://linkinghub.elsevier.com/retrieve/pii/S0145212696001087.
- ^ Lee, Tae-Jin; Jung, Eun Mi; Lee, Jung Tae; Kim, Shin; Park, Jong-Wook; Choi, Kyeong Sook; Kwon, Taeg Kyu (2006). "Mithramycin a sensitizes cancer cells to TRAIL-mediated apoptosis by down-regulation of XIAP gene promoter through Sp1 sites". Molecular Cancer Therapeutics 5 (11): 2737–46. doi:10.1158/1535-7163.MCT-06-0426. PMID 17121920.
- ^ Lin, Ruo-Kai; Hsu, Chun-Hua; Wang, Yi-Ching (2007). "Mithramycin a inhibits DNA methyltransferase and metastasis potential of lung cancer cells". Anti-Cancer Drugs 18 (10): 1157–64. doi:10.1097/CAD.0b013e3282a215e9. PMID 17893516.
- ^ Majee, Sangita; Chakrabarti, Abhijit (1999). "Membrane interaction of an antitumor antibiotic, mithramycin, with anionic phospholipid vesicles". Biochemical Pharmacology 57 (9): 981–7. doi:10.1016/S0006-2952(98)00374-8. PMID 10796068.

This antineoplastic or immunomodulatory drug article is a stub. You can help Wikipedia by expanding it. - InChI=1S/C52H76O24/c1-18-29(72-34-14-30(43(58)21(4)68-34)73-33-13-28(54)42(57)20(3)67-33)12-26-10-25-11-27(49(66-9)48(63)41(56)19(2)53)50(47(62)39(25)46(61)38(26)40(18)55)76-36-16-31(44(59)23(6)70-36)74-35-15-32(45(60)22(5)69-35)75-37-17-52(8,65)51(64)24(7)71-37/h10,12,19-24,27-28,30-37,41-45,49-51,53-61,64-65H,11,13-17H2,1-9H3/t19-,20-,21-,22-,23-,24-,27?,28-,30-,31-,32-,33+,34+,35+,36+,37+,41+,42-,43+,44-,45-,49+,50+,51-,52+/m1/s1
