- Mitoxantrone
-
Mitoxantrone 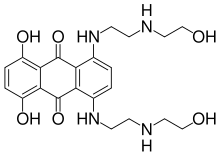
Systematic (IUPAC) name 1,4-dihydroxy-5,8-bis[2-(2-hydroxyethylamino)
ethylamino]-anthracene-9,10-dioneClinical data Trade names Novantrone AHFS/Drugs.com monograph MedlinePlus a608019 Pregnancy cat. D(US) Legal status ℞ Prescription only Routes Exclusively intravenous Pharmacokinetic data Bioavailability n/a Protein binding 78% Metabolism Hepatic (CYP2E1) Half-life 75 hours Excretion Renal Identifiers CAS number 65271-80-9 
ATC code L01DB07 PubChem CID 4212 DrugBank APRD00371 ChemSpider 4067 
UNII BZ114NVM5P 
KEGG D08224 
ChEBI CHEBI:50729 
ChEMBL CHEMBL58 
Chemical data Formula C22H28N4O6 Mol. mass 444.481 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Mitoxantrone is an anthracenedione (not an anthracycline) antineoplastic agent.
Contents
Uses
It is used in the treatment of certain types of cancer, mostly metastatic breast cancer, acute myeloid leukemia, and non-Hodgkin's lymphoma. It was also shown to improve the survival of children suffering from first relapse of acute lymphoblastic leukaemia.[1]
The combination of mitoxantrone and prednisone is approved as a second-line treatment for metastatic hormone-refractory prostate cancer. This combination has been the first line of treatment, until recently, when combination of docetaxel and prednisone has been shown to improve survival and disease-free period.[2]
Mitoxantrone is also used to treat multiple sclerosis (MS), most notably the subset known as secondary progressive MS. Mitoxantrone will not cure multiple sclerosis, but is effective in slowing the progression of secondary progressive MS and extending the time between relapses in relapsing-remitting MS and progressive relapsing MS.[3]Mechanism of action
Mitoxantrone is a type II topoisomerase inhibitor; it disrupts DNA synthesis and DNA repair in both healthy cells and cancer cells.
It also engages in intercalation.[4]
Side effects
As other drugs in its class, mitoxantrone may cause several adverse reactions of varying severity, such as nausea, vomiting, hair loss, heart damage, and immunosuppression. Some side effects may have delayed onset. Cardiomyopathy is a particularly concerning effect as it is irreversible; regular monitoring with echocardiograms or MUGA scans is recommended for people taking mitoxantrone.
The medication carries a total lifetime dose based on body surface area.[3]
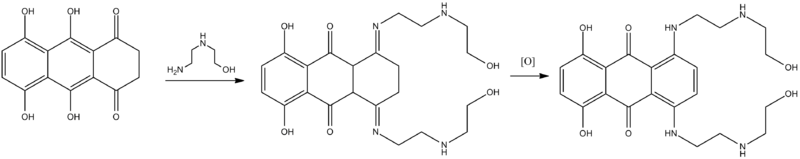
Synthesis
Murdock, K. C.; Child, R. G.; Fabio, P. F.; Angier, Robert D.; Wallace, Roslyn E.; Durr, Frederick E.; Citarella, R. V. (1979). "Antitumor agents. 1. 1,4-Bis[(aminoalkyl)amino]-9,10-anthracenediones". Journal of Medicinal Chemistry 22 (9): 1024. doi:10.1021/jm00195a002. PMID 490545.
See also
- Pixantrone, a mitoxantrone analogue under development
- Naphtoquinoxalinediones, potential antitumorals, obtained from diamino-1,2 anthraquinones using a regioselective synthesis.[5]
- ametantrone
- piroxantrone
References
- ^ Parker C, Waters R, Leighton C, Hancock J, Sutton R, Moorman AV, Ancliff P, Morgan M, Masurekar A, Goulden N, Green N, Révész T, Darbyshire P, Love S, Saha V (2010). "Effect of mitoxantrone on outcome of children with first relapse of acute lymphoblastic leukaemia (ALL R3): an open-label randomised trial". Lancet 376 (9757): 2009–2017. doi:10.1016/S0140-6736(10)62002-8. PMC 3010035. PMID 21131038. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3010035.
- ^ Katzung, Bertram G. (2006). "Cancer Chemotherapy". Basic and clinical pharmacology (10th ed.). New York: McGraw-Hill Medical Publishing Division. ISBN 0-07-145153-6. OCLC 157011367.
- ^ a b Fox E (2006). "Management of worsening multiple sclerosis with mitoxantrone: a review". Clin Ther 28 (4): 461–74. doi:10.1016/j.clinthera.2006.04.013. PMID 16750460.
- ^ Mazerski J, Martelli S, Borowski E (1998). "The geometry of intercalation complex of antitumor mitoxantrone and ametantrone with DNA: molecular dynamics simulations". Acta Biochim. Pol. 45 (1): 1–11. PMID 9701490.
- ^ Baron M, Giorgi-Renault S, Renault J, et al. (1984). "Heterocycles with a quinone function.5.An abnormal reaction of butanedione with 1,2-diaminoanthraquinone - Crystalline structure obtained from naphto(2,3-f) quinoxaline-7,12 dione" (in French). Can. J. Chem. 62 (3): 526–530. doi:10.1139/v84-087. http://www.nrcresearchpress.com/doi/abs/10.1139/v84-087.
Categories:- Topoisomerase inhibitors
- IARC Group 2B carcinogens
- Synthetic phenolic drugs
- Alcohols
Wikimedia Foundation. 2010.