- Vinorelbine
-
Vinorelbine 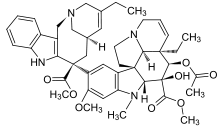
Systematic (IUPAC) name 4-(acetyloxy)- 6,7-didehydro- 15-((2R,6R,8S)-4-ethyl- 1,3,6,7,8,9-hexahydro- 8-(methoxycarbonyl)- 2,6-methano- 2H-azecino(4,3-b)indol-8-yl)- 3-hydroxy- 16-methoxy- 1-methyl- methyl ester, Clinical data Trade names Navelbine AHFS/Drugs.com monograph MedlinePlus a695013 Pregnancy cat. D(AU) D(US) Legal status POM (UK) ℞-only (US) Routes intravenous, oral Pharmacokinetic data Bioavailability 43 ± 14% (oral)[1] Protein binding 79 to 91% Metabolism Hepatic (CYP3A4-mediated) Half-life 27.7 to 43.6 hours Excretion Fecal (46%) and renal (18%) Identifiers CAS number 71486-22-1 
ATC code L01CA04 PubChem CID 5311497 DrugBank APRD00101 ChemSpider 4470974 
UNII Q6C979R91Y 
KEGG D08680 
ChEMBL CHEMBL607994 
Chemical data Formula C45H54N4O8 Mol. mass 778.932 g/mol SMILES eMolecules & PubChem - InChI=1S/C45H54N4O8/c1-8-27-19-28-22-44(40(51)55-6,36-30(25-48(23-27)24-28)29-13-10-11-14-33(29)46-36)32-20-31-34(21-35(32)54-5)47(4)38-43(31)16-18-49-17-12-15-42(9-2,37(43)49)39(57-26(3)50)45(38,53)41(52)56-7/h10-15,19-21,28,37-39,46,53H,8-9,16-18,22-25H2,1-7H3/t28-,37-,38+,39+,42+,43+,44-,45-/m0/s1

Key:GBABOYUKABKIAF-IELIFDKJSA-N
 (what is this?) (verify)
(what is this?) (verify)Vinorelbine (trade name Navelbine) is an anti-mitotic chemotherapy drug that is given as a treatment for some types of cancer, including breast cancer and non-small cell lung cancer.
Contents
Pharmacology
Vinorelbine is the first 5´NOR semi-synthetic vinca alkaloid. It is obtained by semi-synthesis from alkaloids extracted from the rosy periwinkle, Catharanthus roseus.
History
Vinorelbine was invented by the pharmacist Pierre Potier and his team from the CNRS in France in the 1980s and was licensed to the oncology department of the Pierre Fabre Group. The drug was approved in France in 1989 under the brand name Navelbine for the treatment of non-small cell lung cancer. It gained approval to treat metastatic breast cancer in 1991. Vinorelbine received approval by the United States Food and Drug Administration (FDA) in December 1994 sponsored by Burroughs Wellcome Company. Pierre Fabre Group now markets Navelbine in the U.S., where the drug went generic in February 2003.
In most European countries, vinorelbine is approved to treat non-small cell lung cancer and breast cancer.
Uses
As stated above, Vinorelbine is approved for the treatment of Non small cell lung cancer and Metastatic breast cancer. It is also active in rhabdomyosarcoma.[2]
Oral formulation
An oral formulation has been marketed and registered in most European countries for the same settings. It has similar efficacy as the intravenous formulation, avoids venous toxicities of an infusion and is easier to take.
Side effects
Vinorelbine has a number of side-effects that can limit its use:
Lowered resistance to infection, bruising or bleeding, anaemia, constipation, diarrhoea, nausea, numbness or tingling in hands or feet (peripheral neuropathy), tiredness and a general feeling of weakness (asthenia), inflammation of the vein into which it was injected (phlebitis). Seldom severe hyponatremia is seen.
Less common effects are hair loss and allergic reaction.
References
- ^ Marty M, Fumoleau P, Adenis A, Rousseau Y, Merrouche Y, Robinet G, Senac I, Puozzo C (2001). "Oral vinorelbine pharmacokinetics and absolute bioavailability study in patients with solid tumors". Ann Oncol 12 (11): 1643–9. doi:10.1023/A:1013180903805. PMID 11822766.
- ^ Casanova, M; Ferrari, A; Spreafico, F; Terenziani, M; Massimino, M; Luksch, R; Cefalo, G; Polastri, D et al. (2002). "Vinorelbine in previously treated advanced childhood sarcomas: Evidence of activity in rhabdomyosarcoma". Cancer 94 (12): 3263–8. doi:10.1002/cncr.10600. PMID 12115359.
Categories:- Alkaloids
- Mitotic inhibitors
- Acetate esters
- InChI=1S/C45H54N4O8/c1-8-27-19-28-22-44(40(51)55-6,36-30(25-48(23-27)24-28)29-13-10-11-14-33(29)46-36)32-20-31-34(21-35(32)54-5)47(4)38-43(31)16-18-49-17-12-15-42(9-2,37(43)49)39(57-26(3)50)45(38,53)41(52)56-7/h10-15,19-21,28,37-39,46,53H,8-9,16-18,22-25H2,1-7H3/t28-,37-,38+,39+,42+,43+,44-,45-/m0/s1
Wikimedia Foundation. 2010.
