- Mitomycin
-
Mitomycin 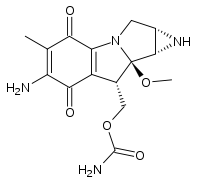 [6-Amino-8a-methoxy-5-methyl-4,7-dioxo-1,1a,2,4,7,8,8a,8b-octahydroazireno[2',3':3,4]pyrrolo[1,2-a]indol-8-yl]methyl carbamateSystematic name{11-Amino-7-methoxy-12-methyl-10,13-dioxo-2,5-diazatetracyclo[7.4.0.02,7.04,6]trideca-1(9),11-dien-8-yl}methyl carbamateOther namesMitomycin C
[6-Amino-8a-methoxy-5-methyl-4,7-dioxo-1,1a,2,4,7,8,8a,8b-octahydroazireno[2',3':3,4]pyrrolo[1,2-a]indol-8-yl]methyl carbamateSystematic name{11-Amino-7-methoxy-12-methyl-10,13-dioxo-2,5-diazatetracyclo[7.4.0.02,7.04,6]trideca-1(9),11-dien-8-yl}methyl carbamateOther namesMitomycin CIdentifiers CAS number 50-07-7 
PubChem 5746  , 44286993 (7R)
, 44286993 (7R)  , 16757880 (7S)
, 16757880 (7S) 
ChemSpider 5544  , 23136133 (7R)
, 23136133 (7R) 
UNII 50SG953SK6 
DrugBank DB00305 KEGG C06681 
ChEBI CHEBI:27504 
ChEMBL CHEMBL105 
ATC code L01 Beilstein Reference 3570056 3DMet B02086 Jmol-3D images Image 1
Image 2- COC12C3NC3CN1C1=C(C2COC(N)=O)C(=O)C(N)=C(C)C1=O
CC1=C(C(=O)C2=C(C1=O)N3C[C@H]4[C@@H]([C@@]3([C@@H]2COC(=O)N)OC)N4)N
- InChI=1S/C15H18N4O5/c1-5-9(16)12(21)8-6(4-24-14(17)22)15(23-2)13-7(18-13)3-19(15)10(8)11(5)20/h6-7,13,18H,3-4,16H2,1-2H3,(H2,17,22)/t6-,7+,13+,15-/m1/s1

Key: NWIBSHFKIJFRCO-WUDYKRTCSA-N
InChI=1S/C15H18N4O5/c1-5-9(16)12(21)8-6(4-24-14(17)22)15(23-2)13-7(18-13)3-19(15)10(8)11(5)20/h6-7,13,18H,3-4,16H2,1-2H3,(H2,17,22)/t6-,7+,13+,15-/m1/s1
Properties Molecular formula C15H18N4O5 Molar mass 334.33 g mol−1 Exact mass 334.127719706 g mol-1 Appearance White or colourless solid Melting point 360 °C, 633 K, 680 °F (low of range)
Solubility in water 8.43 g L-1 log P -1.6 Isoelectric point 10.9 Pharmacology Routes of
administrationEye drops Intravenous Metabolism Hepatic Elimination
half-life8–48 min Legal status Pregnancy
categoryD(AU) D(US)  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references The mitomycins are a family of aziridine-containing natural products isolated from Streptomyces caespitosus or Streptomyces lavendulae.[1] One of these compounds, mitomycin C, finds use as a chemotherapeutic agent by virtue of its antitumour antibiotic activity. It is given intravenously to treat upper gastro-intestinal (e.g. esophageal carcinoma), anal cancers, and breast cancers, as well as by bladder instillation for superficial bladder tumours. It causes delayed bone marrow toxicity and therefore it is usually administered at 6-weekly intervals. Prolonged use may result in permanent bone-marrow damage. It may also cause lung fibrosis and renal damage.
Mitomycin C has also been used topically rather than intravenously in several areas. The first is cancers, particularly bladder cancers and intraperitoneal tumours. It is now well known that a single instillation of this agent within 6 hours of bladder tumor resection can prevent recurrence. The second is in eye surgery where mitomycin c 0.02% is applied topically for 20 seconds to prevent haze after PRK or superlasik. The third is in esophageal and tracheal stenosis where application of mitomycin C onto the mucosa immediately following dilatation will decrease re-stenosis by decreasing the production of fibroblasts and scar tissue.
Mechanism of Action
Mitomycin C is a potent DNA crosslinker. A single crosslink per genome has shown to be effective in killing bacteria. This is accomplished by reductive activation followed by two N-alkylations. Both alkylations are sequence specific for a guanine nucleoside in the sequence 5'-CpG-3'.[2] Potential bis-alkylating heterocylic quinones were synthetised in order to explore their antitumoral activities by bioreductive alkylation.[3] Mitomycin is also used as a chemetherapeutic agent in Glaucoma Surgery
Biosynthesis
In general, the biosynthesis of all mitomycins[4] proceeds via combination of 3-amino-5-hydroxybenzoic acid (AHBA), D-glucosamine, and carbamoyl phosphate, to form the mitosane core, followed by specific tailoring steps. The key intermediate, AHBA, is a common precursor to other anticancer drugs, such as rifamycin and ansamycin.
Specifically, the biosynthesis begins with the addition of phosphoenolpyruvate (PEP) to erythrose-4-phosphate (E4P) with a yet undiscovered enzyme, which is then ammoniated to give 4-amino-3-deoxy-D-arabino heptulosonic acid-7-phosphate (aminoDHAP). Next, DHQ synthase catalyzes a ring closure to give 4-amino3-dehydroquinate (aminoDHQ), which is then undergoes a double oxidation via aminoDHQ dehydratase to give 4-amino-dehydroshikimate (aminoDHS). The key intermediate, 3-amino-5-hydroxybenzoic acid (AHBA), is made via aromatization by AHBA synthase.

Synthesis of the key intermediate, 3-amino-5-hydroxy-benzoic acid.
The mitosane core is synthesized as shown below via condensation of AHBA and D-glucosamine, although no specific enzyme has been characterized that mediates this transformation. Once this condensation has occurred, the mitosane core is tailored by a variety of enzymes. Unfortunately, both the sequence and the identity of these steps are yet to be determined.
- Complete reduction of C-6 - Likely via F420-dependent tetrahydromethanopterin (H4MPT)) reductase and H4MPT:CoM methyltransferase
- Hydroxylation of C-5, C-7 (followed by transamination), and C-9a. - Likely via cytochrome P450 monooxygenase or benzoate hydroxylase
- O-Methylation at C-9a - Likely via SAM dependent methyltransferase
- Oxidation at C-5 and C8 - Unknown
- Intramolecular amination to form aziridine - Unknown
- Carbamoylation at C-10 - Carbamoyl transferrase, with carbamoyl phosphate (C4P) being derived from L-citrulline or L-arginine

References
- ^ Danshiitsoodol N, de Pinho CA, Matoba Y, Kumagai T, Sugiyama M (2006). "The mitomycin C (MMC)-binding protein from MMC-producing microorganisms protects from the lethal effect of bleomycin: crystallographic analysis to elucidate the binding mode of the antibiotic to the protein". J Molec Biol 360 (2): 398–408. doi:10.1016/j.jmb.2006.05.017. PMID 16756991.
- ^ Tomasz, Maria (September 1995). "Mitomycin C: small, fast and deadly (but very selective).". Chemistry and Biology 2 (9): 575–579. doi:10.1016/1074-5521(95)90120-5. PMID 9383461.
- ^ Renault, J.; Baron, M; Mailliet P. & al. Heterocyclic quinones.2.Quinoxaline-5,6-(and 5-8)-diones - Potential antitumoral agents. Eur. J. Med. Chem. 16, 6, 545–550, 1981.
- ^ Mao Y.; Varoglu M.; Sherman D.H. (April 1999). "Molecular characterization and analysis of the biosynthetic gene cluster for the antitumor antibiotic mitomycin C from Streptomyces Iavendulae NRRL 2564.". Chemistry and Biology 6 (4): 251–263. doi:10.1016/S1074-5521(99)80040-4. PMID 10099135.
- Hata, T.; Sano, Y.; Sugawara, R.; Matsumae, A.; Kanamori, K.; Shima, T.; Hoshi, T. J. Antibiot. Ser. A 1956, 9, 141–146.
- Fukuyama, T.; Yang, L. "Total Synthesis of (±)-Mitomycins via Isomitomycin A." J. Am. Chem. Soc. 1987, 109, 7881–7882.
- Mao, Y.; Varoglu, M.; Sherman, D.H. "Molecular characterization and analysis of the biosynthetic cluster for the antitumor antibiotic mitomycin C from Streptomyces lavendulae NRRL 2564." Chemistry & Biology 1999, 6, 251–263.
- Varoglu, M.; Mao, Y.; Sherman, D.H. "Mapping the Biosynthetic Pathway by Functional Analysis of the MitM Aziridine N-Methyltransferase." J. Am. Chem. Soc. 2001, 123, 6712–6713 and references therein.
Categories:- Quinones
- DNA replication inhibitors
- IARC Group 2B carcinogens
- Carbamates
- Ethers
- Aziridines
- COC12C3NC3CN1C1=C(C2COC(N)=O)C(=O)C(N)=C(C)C1=O
Wikimedia Foundation. 2010.
