- Actinomycin
-
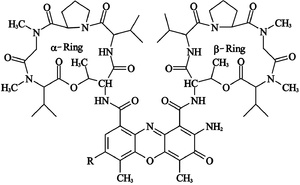
Actinomycin D 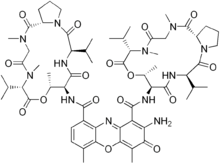
Systematic (IUPAC) name 2-amino-N,N'- bis[(6S,9R,10S,13R,18aS)- 6,13-diisopropyl- 2,5,9-trimethyl- 1,4,7,11,14-pentaoxohexadecahydro- 1H-pyrrolo[2,1-i] [1,4,7,10,13] oxatetraazacyclohexadecin- 10-yl]- 4,6-dimethyl- 3-oxo- 3H-phenoxazine- 1,9-dicarboxamide Clinical data MedlinePlus a682224 Pregnancy cat. ? Legal status ? Pharmacokinetic data Protein binding 5% Half-life 36 hours Identifiers CAS number 50-76-0 
ATC code L01DA01 PubChem CID 2019 DrugBank APRD00124 ChemSpider 10482167 
UNII 1CC1JFE158 
KEGG C06770 
ChEBI CHEBI:27666 
ChEMBL CHEMBL1554 
Synonyms 2-Amino- 4,6-dimethyl- 3-oxo- 3H-phenoxazine- 1,9-dicarboxylic acid bis- [(5,12-diisopropyl- 9,13,16-trimethyl- 4,7,11,14,17-pentaoxo- hexadecahydro- 10-oxa- 3a,6,13,16-tetraaza- cyclopentacyclohexadecen- 8-yl)- amide] Chemical data Formula C62H86N12O16 Mol. mass 1255.42 g/mol - InChI=1S/C62H86N12O16/c1-27(2)42-59(84)73-23-17-19-36(73)57(82)69(13)25-38(75)71(15)48(29(5)6)61(86)88-33(11)44(55(80)65-42)67-53(78)35-22-21-31(9)51-46(35)64-47-40(41(63)50(77)32(10)52(47)90-51)54(79)68-45-34(12)89-62(87)49(30(7)8)72(16)39(76)26-70(14)58(83)37-20-18-24-74(37)60(85)43(28(3)4)66-56(45)81/h21-22,27-30,33-34,36-37,42-45,48-49H,17-20,23-26,63H2,1-16H3,(H,65,80)(H,66,81)(H,67,78)(H,68,79)/t33-,34-,36+,37+,42-,43-,44+,45+,48+,49+/m1/s1

Key:RJURFGZVJUQBHK-IIXSONLDSA-N
 (what is this?) (verify)
(what is this?) (verify)The actinomycins are a class of polypeptide antibiotics isolated from soil bacteria of the genus Streptomyces, of which the most significant is actinomycin D. It was the first antibiotic isolated by Selman Waksman and his co-worker H. B. Woodruff in 1940.[1]
Contents
Mechanism
Actinomycin D is primarily used as an investigative tool in cell biology to inhibit transcription. It does this by binding DNA at the transcription initiation complex and preventing elongation by RNA polymerase.[2] Because it can bind DNA duplexes, it can also interfere with DNA replication, although other chemicals such as hydroxyurea are better suited for use in the laboratory as inhibitors of DNA synthesis.
Clinical use
As chemotherapy
Actinomycin D, also known generically as dactinomycin, was approved by the US FDA on December 10, 1964 and launched by Merck, Sharpe and Dohme under the trade name Cosmegen[3]. It is one of the older chemotherapy drugs, and has been used in therapy for many years.
It is a clear, yellow liquid administered intravenously and most commonly used in treatment of a variety of cancers including gestational trophoblastic neoplasia,[4] Wilms' tumor[5] and rhabdomyosarcoma.[6]
As an antibiotic
It was the first antibiotic shown to have anti-cancer activity, but is not normally used as such, as it is highly toxic, causing damage to genetic material.
Research use
Actinomycin D and its fluorescent derivative, 7-aminoactinomycin D (7-AAD), are used as stains in microscopy and flow cytometry applications. The affinity of these stains/compounds for GC-rich regions of DNA strands makes them excellent markers for DNA. 7-AAD binds to single stranded DNA; therefore it is a useful tool in determining apoptosis and distinguishing between dead cells and live ones.[7]
References
- ^ Waksman S A, Woodruff H B (1940). "Bacteriostatic and bacteriocidal substances produced by soil actinomycetes". Proc Soc Exper Biol 45: 609–614.
- ^ Sobell H (1985). "Actinomycin and DNA transcription". Proc Natl Acad Sci USA 82 (16): 5328–31. doi:10.1073/pnas.82.16.5328. PMC 390561. PMID 2410919. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=390561.
- ^ http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm
- ^ Turan T, Karacay O, Tulunay G, Boran N, Koc S, Bozok S, Kose M (2006). "Results with EMA/CO (etoposide, methotrexate, actinomycin D, cyclophosphamide, vincristine) chemotherapy in gestational trophoblastic neoplasia". Int J Gynecol Cancer 16 (3): 1432–8. doi:10.1111/j.1525-1438.2006.00606.x. PMID 16803542.
- ^ Abd El-Aal H, Habib E, Mishrif M (2005). "Wilms' Tumor: The Experience of the Pediatric Unit of Kasr El-Aini Center of Radiation Oncology and Nuclear Medicine (NEMROCK)". J Egypt Natl Canc Inst 17 (4): 308–11. PMID 17102824.
- ^ Khatua S, Nair C, Ghosh K (2004). "Immune-mediated thrombocytopenia following dactinomycin therapy in a child with alveolar rhabdomyosarcoma: the unresolved issues". J Pediatr Hematol Oncol 26 (11): 777–9. doi:10.1097/00043426-200411000-00020. PMID 15543019.
- ^ 7-Amino-Actinomycin D, Fermentek
External links
Categories:- Polypeptide antibiotics
- DNA replication inhibitors
- Depsipeptides
- InChI=1S/C62H86N12O16/c1-27(2)42-59(84)73-23-17-19-36(73)57(82)69(13)25-38(75)71(15)48(29(5)6)61(86)88-33(11)44(55(80)65-42)67-53(78)35-22-21-31(9)51-46(35)64-47-40(41(63)50(77)32(10)52(47)90-51)54(79)68-45-34(12)89-62(87)49(30(7)8)72(16)39(76)26-70(14)58(83)37-20-18-24-74(37)60(85)43(28(3)4)66-56(45)81/h21-22,27-30,33-34,36-37,42-45,48-49H,17-20,23-26,63H2,1-16H3,(H,65,80)(H,66,81)(H,67,78)(H,68,79)/t33-,34-,36+,37+,42-,43-,44+,45+,48+,49+/m1/s1
Wikimedia Foundation. 2010.