- Mercaptopurine
-
Mercaptopurine 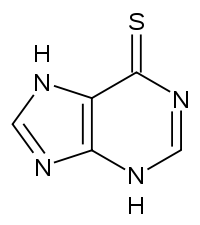
Systematic (IUPAC) name 3,7-dihydropurine-6-thione Clinical data Trade names Purinethol AHFS/Drugs.com monograph MedlinePlus a682653 Pregnancy cat. ?,(Increased Risk of Abortion) Legal status ? Routes Oral Pharmacokinetic data Bioavailability 5 to 37% Metabolism xanthine oxidase Half-life 60 to 120 min., longer for its active metabolites Excretion Renal Identifiers CAS number 50-44-2 
ATC code L01BB02 PubChem CID 667490 DrugBank APRD01096 ChemSpider 580869 
UNII PKK6MUZ20G 
KEGG D04931 
ChEBI CHEBI:50667 
ChEMBL CHEMBL1425 
Chemical data Formula C5H4N4S Mol. mass 152.177 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Mercaptopurine (also called 6-mercaptopurine, 6-MP or its brand name Purinethol) is an immunosuppressive drug.
It is a thiopurine.[1]
Contents
Uses
It is used to treat leukemia, pediatric non-Hodgkin's lymphoma,[citation needed] polycythemia vera,[citation needed] psoriatic arthritis,[citation needed] and inflammatory bowel disease (such as Crohn's disease and ulcerative colitis).[2]
It has demonstrated some in vitro effectiveness against Mycobacterium paratuberculosis.[3]
Mechanisms of action
6-MP ribonucleotide inhibits purine nucleotide synthesis and metabolism. This alters the synthesis and function of RNA and DNA. Mercaptopurine interferes with nucleotide interconversion and glycoprotein synthesis.
Adverse reactions
Some of the adverse reactions of taking mercaptopurine might include diarrhea, nausea, vomiting, loss of appetite, fatigue, stomach/abdominal pain, weakness, skin rash, darkening of the skin, or hair loss. Serious adverse reactions include mouth sores, fever, sore throat, easy bruising or bleeding, pinpoint red spots on the skin, yellowing of eyes or skin, dark urine, and painful or difficult urination. Unlikely but serious side-effects include: black or tarry stools (melena), bloody stools, and bloody urine.
Symptoms of allergic reaction to mercaptopurine include rash, itching, swelling, dizziness, trouble breathing, and pancreatitis.
Mercaptopurine causes myelosuppression, suppressing the production of white blood cells and red blood cells. It may be toxic to bone marrow. Weekly blood counts are recommended for patients on mercaptopurine. The patient should stop taking the medication at least temporarily if there is an unexplained, abnormally large drop in white blood cell count, or any other blood count.
Patients exhibiting myelosuppression or bone marrow toxicity should be tested for thiopurine methyltransferase (TPMT) enzyme deficiency. Patients with TPMT deficiency are much more likely to develop dangerous myelosuppression. In such patients, it may be possible to continue using mercaptopurine, but at a lower dose.
Drug interactions
Allopurinol inhibits xanthine oxidase, the enzyme that breaks down mercaptopurine. Those taking allopurinol (often used to prevent gout) are at risk for mercaptopurine toxicity. The dose should be reduced or allopurinol should be discontinued.
Precautions
Mercaptopurine can lower the body's ability to fight off infection. Those taking mercaptopurine should get permission from a doctor in order to receive immunizations and vaccinations. It is also recommended that, while on the drug, one should avoid those having recently received oral polio vaccine.
This drug is traditionally not recommended during pregnancy, but this issue has been debated, and current evidence indicates that pregnant women on the drug show no increase in fetal abnormalities. However, women receiving mercaptopurine during the first trimester of pregnancy have an increased incidence of miscarriage.[4] Davis et al. 1999 found that mercaptopurine, compared to methotrexate, was ineffective as a single-agent abortifacient; every woman in the mercaptopurine arm of the study had fetal cardiac activity at follow-up (two weeks later) and was given a suction abortion.[5]
Mercaptopurine causes changes to chromosomes in animals and humans, though a study in 1990[6] found that, "while the carcinogenic potential of 6-MP cannot be precluded, it can be only very weak or marginal." Another study in 1999[7] noted an increased risk of developing leukemia when taking large doses of 6-MP together with other cytotoxic drugs.
See also
References
- ^ Sahasranaman S, Howard D, Roy S (August 2008). "Clinical pharmacology and pharmacogenetics of thiopurines". Eur. J. Clin. Pharmacol. 64 (8): 753–67. doi:10.1007/s00228-008-0478-6. ISBN 0022800804786. PMID 18506437.
- ^ Nielsen OH, Vainer B, Rask-Madsen J (November 2001). "Review article: the treatment of inflammatory bowel disease with 6-mercaptopurine or azathioprine". Aliment. Pharmacol. Ther. 15 (11): 1699–708. doi:10.1046/j.1365-2036.2001.01102.x. PMID 11683683. http://www3.interscience.wiley.com/resolve/openurl?genre=article&sid=nlm:pubmed&issn=0269-2813&date=2001&volume=15&issue=11&spage=1699.
- ^ Shin SJ, Collins MT (February 2008). "Thiopurine drugs azathioprine and 6-mercaptopurine inhibit Mycobacterium paratuberculosis growth in vitro". Antimicrob. Agents Chemother. 52 (2): 418–26. doi:10.1128/AAC.00678-07. PMC 2224720. PMID 18070971. http://aac.asm.org/cgi/pmidlookup?view=long&pmid=18070971.
- ^ Nørgård, B.; L. Pedersen, K. Fonager, S. Rasmussen, H. Sørensen (March 2003). "Azathioprine, mercaptopurine and birth outcome: a population-based cohort study". Alimentary pharmacology and therapeutics 17 (6): 827–834. doi:10.1046/j.1365-2036.2003.01537.x. PMID 12641505.
- ^ Davis, Anne R.; Leslie Miller, Hisham Tamimi, and Allen Gown (June 1999). "Methotrexate Compared With Mercaptopurine for Early Induced Abortion". Obstetrics & Gynecology 93 (6): 904–9. doi:10.1016/S0029-7844(98)00569-9. PMID 10362152. http://www.greenjournal.org/cgi/content/full/93/6/904.
- ^ Maekawa, A.; T. Nagaoka, H. Onodera, Y. Matsushima, A. Todate, M. Shibutani, H. Ogasawara, Y. Kodama and Y. Hayashi (May 1990). "Two-year carcinogenicity study of 6-mercaptopurine in F344 rats". Journal of Cancer Research and Clinical Oncology 116 (3): 245–250. doi:10.1007/BF01612898. PMID 2370249. http://www.springerlink.com/content/k28hxj615p761764/.
- ^ Bo J, Schrøder H, Kristinsson J, Madsen B, Szumlanski C, Weinshilboum R, Andersen JB, Schmiegelow K (September 1999). "Possible carcinogenic effect of 6-mercaptopurine on bone marrow stem cells: relation to thiopurine metabolism". Cancer 86 (6): 1080–6. doi:10.1002/(SICI)1097-0142(19990915)86:6<1080::AID-CNCR26>3.0.CO;2-5. PMID 10491537.
External links
Categories:- Antineoplastic antimetabolites
- Purines
- Thiones
Wikimedia Foundation. 2010.
