- Carboplatin
-
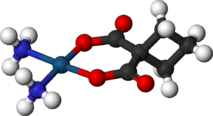
Carboplatin 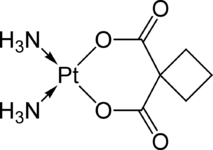
Systematic (IUPAC) name cis-diammine(cyclobutane-1,1-dicarboxylate-O,O')platinum(II) Clinical data Trade names Paraplatin AHFS/Drugs.com monograph MedlinePlus a695017 Pregnancy cat. D(US) Legal status Rx Only Routes Intravenous Pharmacokinetic data Bioavailability complete Protein binding Very low Half-life 1.1-2 hours Excretion hepatic Identifiers CAS number 41575-94-4 
ATC code L01XA02 PubChem CID 498142 DrugBank APRD00466 ChemSpider 8514637 
UNII BG3F62OND5 
KEGG D01363 
ChEBI CHEBI:31355 
ChEMBL CHEMBL288376 
Chemical data Formula C6H12N2O4Pt Mol. mass 371.249 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Carboplatin, or cis-Diammine(1,1-cyclobutanedicarboxylato)platinum(II) (trade names Paraplatin and Paraplatin-AQ) is a chemotherapy drug used against some forms of cancer (mainly ovarian carcinoma, lung, head and neck cancers).[1] It was introduced in the late 1980s and has since gained popularity in clinical treatment due to its vastly reduced side-effects compared to its parent compound cisplatin. Cisplatin and carboplatin, as well as oxaliplatin, interact with DNA, akin to the mechanism of alkylating agents.
Contents
History
Carboplatin was discovered and developed at the Institute of Cancer Research in London. Bristol-Myers Squibb gained Food and Drug Administration (FDA) approval for carboplatin, under the brand name Paraplatin, in March 1989. Starting in October 2004, generic versions of the drug became available.
Pharmacology
Chemistry
In terms of its structure, carboplatin differs from cisplatin in that it has a bidentate dicarboxylate (CBDCA) ligand in place of the two chloride ligand, which are the leaving groups in cisplatin. It exhibits lower reactivity and slower DNA binding kinetics, although it forms the same reaction products in vitro at equivalent doses with cisplatin. Unlike cisplatin, carboplatin may be susceptible to alternative mechanisms. Some results show that cisplatin and carboplatin cause different morphological changes in MCF-7 cell lines while exerting their cytotoxic behaviour. The diminished reactivity limits protein-carboplatin complexes, which are excreted. The lower excretion rate of carboplatin means that more is retained in the body, and hence its effects are longer lasting (a retention half-life of 30 hours for carboplatin, compared to 1.5-3.6 hours in the case of cisplatin).
Mode of action
Two theories exist to explain the molecular mechanism of action of carboplatin with DNA:
- Aquation, or the like-cisplatin hypothesis.
- Activation, or the unlike-cisplatin hypothesis.
The former is more accepted owing to the similarity of the leaving groups with its predecessor cisplatin, while the latter hypothesis envisages a biological activation mechanism to release the active Pt2+ species.
Side-effects
Relative to cisplatin, the greatest benefit of carboplatin is its reduced side effects, particularly the elimination of nephrotoxic effects. Nausea and vomiting are less severe and more easily controlled.
The main drawback of carboplatin is its myelosuppressive effect. This causes the blood cell and platelet output of bone marrow in the body to decrease quite dramatically, sometimes as low as 10% of its usual production levels. The nadir of this myelosuppression usually occurs 21–28 days after the first treatment, after which the blood cell and platelet levels in the blood begin to stabilize, often coming close to its pre-carboplatin levels. This decrease in white blood cells (neutropenia) can cause complications, and is sometimes treated with drugs like filgrastim. The most notable complication of neutropenia is increased probability of infection by opportunistic organisms, which necessitates readmission to hospital and treatment with antibiotics.
Carboplatin is less potent than cisplatin; depending on the strain of cancer, carboplatin may only be 1/8 to 1/45 as effective. The clinical standard of dosage of carboplatin is usually a 4:1 ratio compared to cisplatin; that is, for a dose that usually requires a particular dose of cisplatin, four times more carboplatin is needed to achieve the same effectiveness. The stable property of carboplatin is a mixed blessing: once uptake of the drug occurs, its retention half-life is considerably longer than cisplatin, but it is also this inertness that causes carboplatin to go right through the human body, and up to 90% of the carboplatin given can be recovered in urine.
The effectiveness of carboplatin can be increased by first incubating carboplatin in a sodium chloride (NaCl) solution. After 24 hours, an analysis is performed on the solution by separating the compounds by thin-layer chromatography (TLC). The TLC isolates cisplatin, carboplatin, and several platinum by-products in the solution. Numerous trials have shown a trend that the survival rate of E. coli dropped dramatically as the molarity of the NaCl incubating solution increased. The treated E. coli also showed decreased amounts of alkaline phosphatase, a protein indicator of cellular size. This suggests that as this incubated carboplatin solution is administered to cells, they began to shrink and eventually die; apparently by the same mechanism that cisplatin works.
GemCarbo chemotherapy for lung cancer
GemCarbo chemotherapy (consisting of gemcitabine, also known as Gemzar, and carboplatin) is used to treat several different types of cancer, but is most commonly used to treat lung cancer.[2] GemCarbo chemotherapy is usually conducted as a day patient treatment, involving a blood test the day before. The drugs are administered by infusion. The GemCarbo regimen is given as a 21-day cycle. On the first day of treatment the patient is given both the gemcitabine and carboplatin. On the same day of the following week (day eight) there is a drip of gemcitabine only. There then follows a rest period of two weeks which completes one cycle of chemotherapy. The next cycle of treatment is given after a rest period, ends three weeks after the first injection. Usually 4–6 cycles of treatment are given over a period of 3–4 months to complete a full course of treatment. This treatment may prevent the further spread of the cancer or in some cases may reduce the size of the tumor between 20%-80%, depending upon the individual.
Current events
A recent study in mutant mice suggests that in the subset of women with breast cancer due to BRCA1 and BRCA2 genes (these cause a variety of familial breast cancer) carboplatin may be as much as 20 times more effective than the usual breast cancer treatments.[3] However, similar data in humans has not yet been shown.
Carboplatin has also been used to treat testicular cancer patients with stage 1 seminoma. Recent research indicates that this treatment is more effective and has fewer side effects than adjuvant radiotherapy.[4][5][6] It is as effective as radiotherapy at preventing development of seminoma in the remaining testicle.[7]
References
- ^ Wheate NJ, Walker S, Craig GE, Oun R (September 2010). "The status of platinum anticancer drugs in the clinic and in clinical trials". Dalton Trans 39 (35): 8113–27. doi:10.1039/c0dt00292e. PMID 20593091.
- ^ Macmillan GemCarbo chemotherapy
- ^ Henderson, Mark (May 1, 2006). "Lung cancer drug may fight breast tumour in women". Times Online. http://www.timesonline.co.uk/tol/news/uk/article711744.ece.
- ^ http://www.asco.org/ASCO/Abstracts+%26+Virtual+Meeting/Abstracts?&vmview=abst_detail_view&confID=55&abstractID=34426
- ^ "Testicular cancer drug effective". BBC News. 22 July 2005. http://news.bbc.co.uk/2/hi/health/4700755.stm.
- ^ Rose, David (October 6, 2008). "Chemotherapy drug, carboplatin, ‘is safer cure for testicular cancer’". The Times. http://www.timesonline.co.uk/tol/life_and_style/health/article4887854.ece.
- ^ Nelson, Roxanne (June 10, 2008). "Carboplatin as Effective as Radiation in Preventing Relapse in Testicular Cancer". Medscape Medical News. http://www.medscape.com/viewarticle/575825.
Additional references
- Natarajan G, Malathi R, Holler E (November 1999). "Increased DNA-binding activity of cis-1,1-cyclobutanedicarboxylatodiammineplatinum(II) (carboplatin) in the presence of nucleophiles and human breast cancer MCF-7 cell cytoplasmic extracts: activation theory revisited". Biochem. Pharmacol. 58 (10): 1625–9. doi:10.1016/S0006-2952(99)00250-6. PMID 10535754. http://linkinghub.elsevier.com/retrieve/pii/S0006-2952(99)00250-6.
- Knox RJ, Friedlos F, Lydall DA, Roberts JJ (April 1986). "Mechanism of cytotoxicity of anticancer platinum drugs: evidence that cis-diamminedichloroplatinum(II) and cis-diammine-(1,1-cyclobutanedicarboxylato)platinum(II) differ only in the kinetics of their interaction with DNA". Cancer Res. 46 (4 Pt 2): 1972–9. PMID 3512077. http://cancerres.aacrjournals.org/cgi/pmidlookup?view=long&pmid=3512077.
- Canetta R, Rozencweig M, Carter SK (September 1985). "Carboplatin: the clinical spectrum to date". Cancer Treat. Rev. 12 (Suppl A): 125–36. doi:10.1016/0305-7372(85)90027-1. PMID 3002623.
- Overbeck TL, Knight JM, Beck DJ (April 1996). "A comparison of the genotoxic effects of carboplatin and cisplatin in Escherichia coli". Mutat. Res. 362 (3): 249–59. PMID 8637503.
- Schnurr B, Gust R (August 2002). "Investigations on the decomposition of carboplatin in infusion solutions". Mikrochimica Acta 140 (1–2): 69–76.
- Yang XL, Wang AH (September 1999). "Structural studies of atom-specific anticancer drugs acting on DNA". Pharmacol. Ther. 83 (3): 181–215. doi:10.1016/S0163-7258(99)00020-0. PMID 10576292. http://linkinghub.elsevier.com/retrieve/pii/S0163725899000200.
- Travis LB, Holowaty EJ, Bergfeldt K, et al. (February 1999). "Risk of leukemia after platinum-based chemotherapy for ovarian cancer". N. Engl. J. Med. 340 (5): 351–7. doi:10.1056/NEJM199902043400504. PMID 9929525. http://content.nejm.org/cgi/content/full/340/5/351).
External links
Categories:- Coordination compounds
- Alkylating antineoplastic agents
- Organoplatinum compounds
- Ammine complexes
Wikimedia Foundation. 2010.
