- Carmustine
-
Carmustine 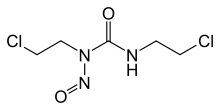
Systematic (IUPAC) name N,N'-bis(2-chloroethyl)-N-nitroso-urea Clinical data AHFS/Drugs.com monograph MedlinePlus a682060 Pregnancy cat. D(US) Legal status ℞ Prescription only Routes Intravenous, wafer for implant Pharmacokinetic data Bioavailability 5 to 28% (oral) Protein binding 80% Metabolism Hepatic (CYP1 A2-mediated) Half-life 15 to 30 min Identifiers CAS number 154-93-8 
ATC code L01AD01 PubChem CID 2578 DrugBank APRD00006 ChemSpider 2480 
UNII U68WG3173Y 
KEGG D00254 
ChEBI CHEBI:3423 
ChEMBL CHEMBL513 
Chemical data Formula C5H9Cl2N3O2 Mol. mass 214.049 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Carmustine or BCNU (bis-chloroethylnitrosourea) is a mustard gas-related β-chloro-nitrosourea compound used as an alkylating agent in chemotherapy. As a dialkylating agent, BCNU is able to form interstrand crosslinks in DNA which prevents DNA replication and DNA transcription.
It has the appearance of a orange-yellow solid.
Carmustine for injection is marketed under the name BiCNU by Bristol-Myers Squibb.
Contents
Uses
It is used in the treatment of several types of brain cancer (including glioma, glioblastoma multiforme, medulloblastoma and astrocytoma), multiple myeloma and lymphoma (Hodgkin's and non-Hodgkin). BCNU is sometimes used in conjunction with alkyl guanine transferase (AGT) inhibitors, such as O6-benzylguanine. The AGT-inhibitors increase the efficacy of BCNU by inhibiting the Direct Reversal pathway of DNA repair, which will prevent formation of the interstrand crosslink between the N1 of guanine and the N3 of cytosine.
Side effects
Bone marrow may take 6 weeks to recover function following treatment with carmustine. Weekly monitoring of platelet and white blood cell counts are recommended as a basis for patient-specific adjustments to dosage regimens. Bone marrow and pulmonary toxicities are a function of lifetime cumulative dose.
Pulmonary toxicity
Pulmonary toxicity characterised by pulmonary infiltrates and/or fibrosis (scarring of the lungs). Cases of fatal pulmonary toxicity have been reported. Delayed onset pulmonary fibrosis
Hematologic toxicity
Delayed myelosuppression. Thrombocytopenia usually occurs about 4 weeks post administration. Leukopenia occurs approximately 5–6 weeks after administration. Cumulative myelosuppression, manifested by more depressed indices. Anemia also occurs, but is less frequent and less severe than thrombocytopenia or leukopenia. The occurrence of acute leukemia and bone marrow dysplasias have been reported in patients following long-term nitrosourea therapy.
Gastrointestinal toxicity
Nausea and vomiting after IV administration are noted frequently. This usually occurs within 2 hours of administration. This is usually dose related. Prior administration of antiemetics is effective in diminishing and sometimes preventing side effects.
Hepatotoxicity
A reversible type of hepatic toxicity, manifested by increased transaminase, alkaline phosphatase and bilirubin levels, has been reported in a small percentage of patients.
Nephrotoxicity
Renal abnormalities consisting of progressive azotemia, decrease in kidney size and renal failure have been reported in patients who received large cumulative doses after prolonged therapy. Kidney damage has also been reported occasionally in patients receiving lower total doses.
Implants
In the treatment of brain tumours, the U.S. Food and Drug Administration (FDA) approved biodegradable discs, Gliadel, infused with carmustine can be used.[1] They are implanted under the skull during a surgery called a craniotomy.[2]
References
- ^ Ewend MG, Brem S, Gilbert M, et al. (June 2007). "Treatment of single brain metastasis with resection, intracavity carmustine polymer wafers, and radiation therapy is safe and provides excellent local control". Clin. Cancer Res. 13 (12): 3637–41. doi:10.1158/1078-0432.CCR-06-2095. PMID 17575228. http://clincancerres.aacrjournals.org/cgi/pmidlookup?view=long&pmid=17575228.
- ^ http://www.cancerbackup.org.uk/Treatments/Chemotherapy/Individualdrugs/Gliadelimplants
External links
- MedlinePlus DrugInfo medmaster-a682060
- BiCNU (package insert; U.S.)

This antineoplastic or immunomodulatory drug article is a stub. You can help Wikipedia by expanding it.