- Cytosine
-
Cytosine 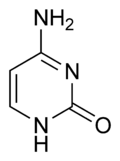

 4-aminopyrimidin-2(1H)-oneOther names4-amino-1H-pyrimidine-2-one
4-aminopyrimidin-2(1H)-oneOther names4-amino-1H-pyrimidine-2-oneIdentifiers CAS number 71-30-7 
PubChem 597 ChemSpider 577 
UNII 8J337D1HZY 
KEGG C00380 
MeSH Cytosine ChEBI CHEBI:16040 
ChEMBL CHEMBL15913 
Jmol-3D images Image 1 - c1cnc(=O)[nH]c1N
Properties Molecular formula C4H5N3O Molar mass 111.10 g/mol Density 1.55 g/cm3 (calculated) Melting point 320-325 °C, 593-598 K, 608-617 °F (decomp.)
Acidity (pKa) 4.45 (secondary), 12.2 (primary)[1]  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Cytosine (C) is one of the four main bases found in DNA and RNA, along with adenine, guanine, and thymine (uracil in RNA). It is a pyrimidine derivative, with a heterocyclic aromatic ring and two substituents attached (an amine group at position 4 and a keto group at position 2). The nucleoside of cytosine is cytidine. In Watson-Crick base pairing, it forms three hydrogen bonds with guanine.
Contents
History
Cytosine was discovered by Albrecht Kossel in 1894 when it was hydrolysed from calf thymus tissues.[2] A structure was proposed in 1903, and was synthesized (and thus confirmed) in the laboratory in the same year.
Cytosine recently found use in quantum computation. The first time any quantum mechanical properties were harnessed to process information took place on August 1st in 1998 when researchers at Oxford implemented David Deutsch's algorithm on a two qubit NMRQC (Nuclear Magnetic Resonance Quantum Computer) based on the cytosine molecule.[3]
Chemical reactions
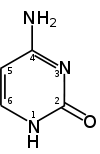 Cytosine with numbered components. Methylation occurs on carbon nr 5.
Cytosine with numbered components. Methylation occurs on carbon nr 5.
Cytosine can be found as part of DNA, as part of RNA, or as a part of a nucleotide. As cytidine triphosphate (CTP), it can act as a co-factor to enzymes, and can transfer a phosphate to convert adenosine diphosphate (ADP) to adenosine triphosphate (ATP).
In DNA and RNA, cytosine is paired with guanine. However, it is inherently unstable, and can change into uracil (spontaneous deamination). This can lead to a point mutation if not repaired by the DNA repair enzymes such as uracil glycosylase, which cleaves a uracil in DNA.
Cytosine can also be methylated into 5-methylcytosine by an enzyme called DNA methyltransferase or be methylated and hydroxylated to make 5-hydroxymethylcytosine. Active enzymatic deamination of cytosine or 5-methylcytosine by the APOBEC family of cytosine deaminases could have both beneficial and detrimental implications on various cellular processes as well as on organismal evolution.[4] The implications of deamination on 5-hydroxymethylcytosine, on the other hand, remains less understood.
References
- ^ Dawson, R.M.C., et al., Data for Biochemical Research, Oxford, Clarendon Press, 1959.
- ^ Kossel, A.; Steudel, H. Z. Physiol. Chem. 1903, 38, 49
- ^ Jones, J.A.; M. Mosca (1998-08-01). "Implementation of a quantum algorithm on a nuclear magnetic resonance quantum computer". J.Chem.Phys 109 (109): 1648–1653. doi:10.1063/1.476739. http://www.citebase.org/abstract?id=oai%3AarXiv.org%3Aquant-ph%2F9801027. Retrieved 2007-10-18.
- ^ Chahwan R., Wontakal S.N., and Roa S. (2010). "Crosstalk between genetic and epigenetic information through cytosine deamination". Trends in Genetics 26 (10): 443–448. doi:10.1016/j.tig.2010.07.005. PMID 20800313.
External links
- EINECS number 200-749-5
- Shapiro R (1999). "Prebiotic cytosine synthesis: a critical analysis and implications for the origin of life". Proc. Natl. Acad. Sci. U.S.A. 96 (8): 4396–401. doi:10.1073/pnas.96.8.4396. PMC 16343. PMID 10200273. http://www.pnas.org/cgi/content/full/96/8/4396.
Nucleic acid constituents Nucleobase Purine (Adenine, Guanine, Purine analogue) · Pyrimidine (Uracil, Thymine, Cytosine, Pyrimidine analogue)Nucleoside Nucleotide
(Nucleoside monophosphate)Nucleoside diphosphate Nucleoside triphosphate biochemical families: prot · nucl · carb (glpr, alco, glys) · lipd (fata/i, phld, strd, gllp, eico) · amac/i · ncbs/i · ttpy/i Categories:- Amines
- Pyrimidones
Wikimedia Foundation. 2010.
