- Guanosine triphosphate
-
Guanosine triphosphate 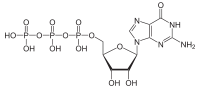 [(2R,3S,4R,5R)-5-(2-amino-6-oxo-3H-purin-9-yl)-3,4- dihydroxyoxolan-2-yl]methyl (hydroxy-phosphonooxyphosphoryl) hydrogen phosphateOther namesguanosine triphosphate, 9-β-D-ribofuranosylguanine-5'-triphosphate, 9-β-D-ribofuranosyl-2-amino-6-oxo-purine-5'-triphosphate
[(2R,3S,4R,5R)-5-(2-amino-6-oxo-3H-purin-9-yl)-3,4- dihydroxyoxolan-2-yl]methyl (hydroxy-phosphonooxyphosphoryl) hydrogen phosphateOther namesguanosine triphosphate, 9-β-D-ribofuranosylguanine-5'-triphosphate, 9-β-D-ribofuranosyl-2-amino-6-oxo-purine-5'-triphosphateIdentifiers CAS number 86-01-1 
PubChem 6830 ChemSpider 6569 
MeSH Guanosine+triphosphate ChEBI CHEBI:15996 
IUPHAR ligand 1742 Jmol-3D images Image 1 - c1nc2c(n1[C@H]3[C@@H]([C@@H]([C@H](O3)CO[P@@](=O)(O)O[P@](=O)(O)OP(=O)(O)O)O)O)[nH]c(nc2=O)N
- InChI=1S/C10H16N5O14P3/c11-10-13-7-4(8(18)14-10)12-2-15(7)9-6(17)5(16)3(27-9)1-26-31(22,23)29-32(24,25)28-30(19,20)21/h2-3,5-6,9,16-17H,1H2,(H,22,23)(H,24,25)(H2,19,20,21)(H3,11,13,14,18)/t3-,5-,6-,9-/m1/s1

Key: XKMLYUALXHKNFT-UUOKFMHZSA-N
InChI=1/C10H16N5O14P3/c11-10-13-7-4(8(18)14-10)12-2-15(7)9-6(17)5(16)3(27-9)1-26-31(22,23)29-32(24,25)28-30(19,20)21/h2-3,5-6,9,16-17H,1H2,(H,22,23)(H,24,25)(H2,19,20,21)(H3,11,13,14,18)/t3-,5-,6-,9-/m1/s1
Key: XKMLYUALXHKNFT-UUOKFMHZBF
Properties Molecular formula C10H16N5O14P3 Molar mass 523.18 g mol−1  triphosphate (verify) (what is:
triphosphate (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Guanosine-5'-triphosphate (GTP) is a purine nucleoside triphosphate. It can act as a substrate for the synthesis of RNA during the transcription process. Its structure is similar to that of the guanine nucleobase, the only difference being that nucleotides like GTP have a ribose sugar and three phosphates, with the nucleobase attached to the 1' and the triphosphate moiety attached to the 5' carbons of the ribose.
It also has the role of a source of energy or an activator of substrates in metabolic reactions, like that of ATP, but more specific. It is used as a source of energy for protein synthesis.
GTP is essential to signal transduction, particularly with G-proteins, in second-messenger mechanisms where it is converted to GDP (guanosine diphosphate) through the action of GTPases.
Contents
Uses
Energy Transfer
GTP is involved in energy transfer within the cell. For instance, a GTP molecule is generated by one of the enzymes in the citric acid cycle. This is tantamount to the generation of one molecule of ATP, since GTP is readily converted to ATP.[1]
Genetic Translation
During the elongation stage of translation, GTP is used as an energy source for the binding of a new amino-bound tRNA to the A site of the ribosome. GTP is also used as an energy source for the translocation of the ribosome towards the 3' end of the mRNA.[2]
Microtubule Dynamic Instability
During microtubule polymerization, each heterodimer formed by an alpha and a beta tubulin molecule carries two GTP molecules, and the GTP is hydrolyzed to GDP when the tubulin dimers are added to the plus end of the growing microtubule. Such GTP hydrolysis is not mandatory for microtubule formation, but it appears that only GDP-bound tubulin molecules are able to depolymerize. Thus, a GTP-bound tubulin serves as a cap at the tip of microtubule to protect from depolymerization; and once the GTP is hydrolyzed, the microtubule begins to depolymerize and shrink rapidly.[3]
cGTP
Cyclic guanosine triphosphate (cGTP) helps cyclic adenosine monophosphate (cAMP) activate cyclic nucleotide-gated ion channels in the olfactory system.[4]
See also
- GTP-gamma-S
- G protein
- G protein coupled receptor
- 5'-Guanylyl imidodiphosphate
References
- ^ Berg, JM; JL Tymoczko, L Stryer (2002). Biochemistry (5th ed.). WH Freeman and Company. pp. 476. ISBN 0-7167-4684-0.
- ^ Solomon, EP; LR Berg, DW Martin (2005). Biology (7th ed.). pp. 244–245.
- ^ Microtubule structure© text copyright 1996 Gwen V. Childs, Ph.D.
- ^ Medical Physiology, Boron & Boulpaep, ISBN 1-4160-2328-3, Elsevier Saunders 2005. Updated edition. Page 90.
Nucleic acid constituents Nucleobase Nucleoside Nucleotide
(Nucleoside monophosphate)Nucleoside diphosphate Nucleoside triphosphate biochemical families: prot · nucl · carb (glpr, alco, glys) · lipd (fata/i, phld, strd, gllp, eico) · amac/i · ncbs/i · ttpy/iCategories:- Nucleotides
- Purines
Wikimedia Foundation. 2010.
