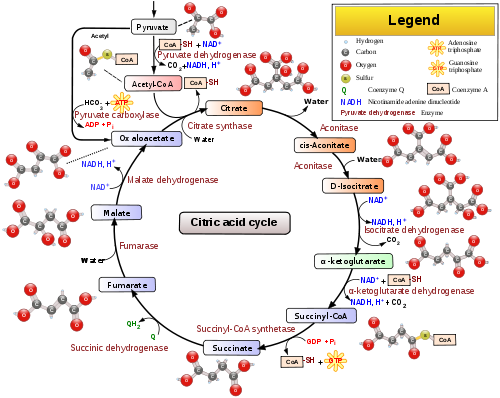
- Citric acid cycle
-
The citric acid cycle — also known as the tricarboxylic acid cycle (TCA cycle), the Krebs cycle, or the Szent-Györgyi-Krebs cycle[1][2] — is a series of chemical reactions which is used by all aerobic living organisms to generate energy through the oxidization of acetate derived from carbohydrates, fats and proteins into carbon dioxide and water. In addition, the cycle provides precursors for the biosynthesis of compounds including certain amino acids as well as the reducing agent NADH that is used in numerous biochemical reactions. Its central importance to many biochemical pathways suggests that it was one of the earliest established components of cellular metabolism and may have originated abiogenically.[3]
The name of this metabolic pathway is derived from citric acid (a type of tricarboxylic acid) which is first consumed and then regenerated by this sequence of reactions to complete the cycle. In addition, the cycle consumes acetate in the form of acetyl-CoA, reduces NAD+ to NADH, and produces carbon dioxide. The NADH generated by the TCA cycle is fed into the oxidative phosphorylation pathway. The net result of these two closely linked pathways is the oxidation of nutrients to produce energy in the form of ATP.
In eukaryotic cells, the citric acid cycle occurs in the matrix of the mitochondrion. Bacteria also use the TCA cycle to generate energy, but since they lack mitochondria, the reaction sequence is performed in the cytosol.
The components and reactions of the citric acid cycle were established in the 1930s by seminal work from the Nobel laureates Albert Szent-Györgyi[4] and Hans Adolf Krebs.[5]
Contents
Evolution
Components of the TCA cycle were derived from anaerobic bacteria and the TCA cycle itself may have evolved more than once.[6] Theoretically there are several alternatives to the TCA cycle, however the TCA cycle appears to be the most efficient.[7] If several alternatives independently evolved, they all undoubtedly rapidly converged to the TCA cycle.
Overview
The citric acid cycle is a key component of the metabolic pathway by which all aerobic organisms generate energy. Through catabolism of sugars, fats, and proteins, a two carbon organic product acetate in the form of acetyl-CoA is produced. Acetyl-CoA along with two equivalents of water (H2O) are consumed by the citric acid cycle producing two equivalents of carbon dioxide (CO2) and one equivalent of HS-CoA. In addition, one complete turn of the cycle converts three equivalents of nicotinamide adenine dinucleotide (NAD+) into three equivalents of reduced NAD+ (NADH), one equivalent of ubiquinone (Q) of into one equivalent of reduced ubiquinone (QH2), and one equivalent each of guanosine diphosphate (GDP) and inorganic phosphate (Pi) into one equivalent of guanosine triphosphate (GTP). The NADH and QH2 that is generated by the citric acid cycle is in turn used by the oxidative phosphorylation pathway to generate energy rich adenosine triphosphate (ATP).
One of the primary sources of acetyl-CoA are sugars that are broken down by glycolysis to produce pyruvate that in turn is decarboxylated by the enzyme pyruvate dehydrogenase generating acetyl-CoA according to the following reaction scheme:
- CH3C(=O)C(=O)O– (pyruvate) + HSCoA + NAD+ → CH3C(=O)SCoA (acetyl-CoA) + NADH + H+ + CO2
The product of this reaction, acetyl-CoA, is the starting point for the citric acid cycle. Below is a schematic outline of the cycle:
- The citric acid cycle begins with the transfer of a two-carbon acetyl group from acetyl-CoA to the four-carbon acceptor compound (oxaloacetate) to form a six-carbon compound (citrate).
- The citrate then goes through a series of chemical transformations, losing two carboxyl groups as CO2. The carbons lost as CO2 originate from what was oxaloacetate, not directly from acetyl-CoA. The carbons donated by acetyl-CoA become part of the oxaloacetate carbon backbone after the first turn of the citric acid cycle. Loss of the acetyl-CoA-donated carbons as CO2 requires several turns of the citric acid cycle. However, because of the role of the citric acid cycle in anabolism, they may not be lost, since many TCA cycle intermediates are also used as precursors for the biosynthesis of other molecules.[8]
- Most of the energy made available by the oxidative steps of the cycle is transferred as energy-rich electrons to NAD+, forming NADH. For each acetyl group that enters the citric acid cycle, three molecules of NADH are produced.
- Electrons are also transferred to the electron acceptor Q, forming QH2.
- At the end of each cycle, the four-carbon oxaloacetate has been regenerated, and the cycle continues.
Steps
Two carbon atoms are oxidized to CO2, the energy from these reactions being transferred to other metabolic processes by GTP (or ATP), and as electrons in NADH and QH2. The NADH generated in the TCA cycle may later donate its electrons in oxidative phosphorylation to drive ATP synthesis; FADH2 is covalently attached to succinate dehydrogenase, an enzyme functioning both in the TCA cycle and the mitochondrial electron transport chain in oxidative phosphorylation. FADH2, therefore, facilitates transfer of electrons to coenzyme Q, which is the final electron acceptor of the reaction catalyzed by the Succinate:ubiquinone oxidoreductase complex, also acting as an intermediate in the electron transport chain.[9]
The citric acid cycle is continuously supplied with new carbon in the form of acetyl-CoA, entering at step 1 below.[10]
Substrates Products Enzyme Reaction type Comment 1 Oxaloacetate +
Acetyl CoA +
H2OCitrate +
CoA-SHCitrate synthase Aldol condensation irreversible,
extends the 4C oxaloacetate to a 6C molecule2 Citrate cis-Aconitate +
H2OAconitase Dehydration reversible isomerisation 3 cis-Aconitate +
H2OIsocitrate Hydration 4 Isocitrate +
NAD+Oxalosuccinate +
NADH + H +Isocitrate dehydrogenase Oxidation generates NADH (equivalent of 2.5 ATP) 5 Oxalosuccinate α-Ketoglutarate +
CO2Decarboxylation rate-limiting, irreversible stage,
generates a 5C molecule6 α-Ketoglutarate +
NAD+ +
CoA-SHSuccinyl-CoA +
NADH + H+ +
CO2α-Ketoglutarate dehydrogenase Oxidative
decarboxylationirreversible stage,
generates NADH (equivalent of 2.5 ATP),
regenerates the 4C chain (CoA excluded)7 Succinyl-CoA +
GDP + PiSuccinate +
CoA-SH +
GTPSuccinyl-CoA synthetase substrate-level phosphorylation or ADP→ATP instead of GDP→GTP,[9]
generates 1 ATP or equivalent8 Succinate +
ubiquinone (Q)Fumarate +
ubiquinol (QH2)Succinate dehydrogenase Oxidation uses FAD as a prosthetic group (FAD→FADH2 in the first step of the reaction) in the enzyme,[9]
generates the equivalent of 1.5 ATP9 Fumarate +
H2OL-Malate Fumarase Hydration 10 L-Malate +
NAD+Oxaloacetate +
NADH + H+Malate dehydrogenase Oxidation reversible (in fact, equilibrium favors malate), generates NADH (equivalent of 2.5 ATP) Mitochondria in animals, including humans, possess two succinyl-CoA synthetases: one that produces GTP from GDP, and another that produces ATP from ADP.[11] Plants have the type that produces ATP (ADP-forming succinyl-CoA synthetase).[10] Several of the enzymes in the cycle may be loosely-associated in a multienzyme protein complex within the mitochondrial matrix.[12]
The GTP that is formed by GDP-forming succinyl-CoA synthetase may be utilized by nucleoside-diphosphate kinase to form ATP (the catalyzed reaction is GTP + ADP → GDP + ATP).[9]
Products
Products of the first turn of the cycle are: one GTP (or ATP), three NADH, one QH2, two CO2.
Because two acetyl-CoA molecules are produced from each glucose molecule, two cycles are required per glucose molecule. Therefore, at the end of two cycles, the products are: two GTP, six NADH, two QH2, and four CO2
Description Reactants Products The sum of all reactions in the citric acid cycle is: Acetyl-CoA + 3 NAD+ + Q + GDP + Pi + 2 H2O → CoA-SH + 3 NADH + 3 H+ + QH2 + GTP + 2 CO2 Combining the reactions occurring during the pyruvate oxidation with those occurring during the citric acid cycle, the following overall pyruvate oxidation reaction is obtained: Pyruvate ion + 4 NAD+ + Q + GDP + Pi + 2 H2O → 4 NADH + 4 H+ + QH2 + GTP + 3 CO2 Combining the above reaction with the ones occurring in the course of glycolysis, the following overall glucose oxidation reaction (excluding reactions in the respiratory chain) is obtained: Glucose + 10 NAD+ + 2 Q + 2 ADP + 2 GDP + 4 Pi + 2 H2O → 10 NADH + 10 H+ + 2 QH2 + 2 ATP + 2 GTP + 6 CO2 The above reactions are balanced if Pi represents the H2PO4- ion, ADP and GDP the ADP2- and GDP2- ions, respectively, and ATP and GTP the ATP3- and GTP3- ions, respectively.
The total number of ATP obtained after complete oxidation of one glucose in glycolysis, citric acid cycle, and oxidative phosphorylation is estimated to be between 30 and 38. A recent assessment of the total ATP yield with the updated proton-to-ATP ratios provides an estimate of 29.85 ATP per glucose molecule.[13]
Regulation
Although pyruvate dehydrogenase is not technically a part of the citric acid cycle, its regulation is included here.
The regulation of the TCA cycle is largely determined by substrate availability and product inhibition. NADH, a product of all dehydrogenases in the TCA cycle with the exception of succinate dehydrogenase, inhibits pyruvate dehydrogenase, isocitrate dehydrogenase, α-ketoglutarate dehydrogenase, and also citrate synthase. Acetyl-coA inhibits pyruvate dehydrogenase, while succinyl-CoA inhibits succinyl-CoA synthetase and citrate synthase. When tested in vitro with TCA enzymes, ATP inhibits citrate synthase and α-ketoglutarate dehydrogenase; however, ATP levels do not change more than 10% in vivo between rest and vigorous exercise. There is no known allosteric mechanism that can account for large changes in reaction rate from an allosteric effector whose concentration changes less than 10%.[14]
Calcium is used as a regulator. It activates pyruvate dehydrogenase, isocitrate dehydrogenase and α-ketoglutarate dehydrogenase.[15] This increases the reaction rate of many of the steps in the cycle, and therefore increases flux throughout the pathway.
Citrate is used for feedback inhibition, as it inhibits phosphofructokinase, an enzyme involved in glycolysis that catalyses formation of fructose 1,6-bisphosphate,a precursor of pyruvate. This prevents a constant high rate of flux when there is an accumulation of citrate and a decrease in substrate for the enzyme.
Recent work has demonstrated an important link between intermediates of the citric acid cycle and the regulation of hypoxia-inducible factors (HIF). HIF plays a role in the regulation of oxygen homeostasis, and is a transcription factor that targets angiogenesis, vascular remodeling, glucose utilization, iron transport and apoptosis. HIF is synthesized consititutively, and hydroxylation of at least one of two critical proline residues mediates their interaction with the von Hippel Lindau E3 ubiquitin ligase complex, which targets them for rapid degradation. This reaction is catalysed by prolyl 4-hydroxylases. Fumarate and succinate have been identified as potent inhibitors of prolyl hydroxylases, thus leading to the stabilisation of HIF.[16]
Major metabolic pathways converging on the TCA cycle
Several catabolic pathways converge on the TCA cycle. Reactions that form intermediates of the TCA cycle in order to replenish them (especially during the scarcity of the intermediates) are called anaplerotic reactions.
The citric acid cycle is the third step in carbohydrate catabolism (the breakdown of sugars). Glycolysis breaks glucose (a six-carbon-molecule) down into pyruvate (a three-carbon molecule). In eukaryotes, pyruvate moves into the mitochondria. It is converted into acetyl-CoA by decarboxylation and enters the citric acid cycle.
In protein catabolism, proteins are broken down by proteases into their constituent amino acids. The carbon backbone of these amino acids can become a source of energy by being converted to acetyl-CoA and entering into the citric acid cycle.
In fat catabolism, triglycerides are hydrolyzed to break them into fatty acids and glycerol. In the liver the glycerol can be converted into glucose via dihydroxyacetone phosphate and glyceraldehyde-3-phosphate by way of gluconeogenesis. In many tissues, especially heart tissue, fatty acids are broken down through a process known as beta oxidation, which results in acetyl-CoA, which can be used in the citric acid cycle. Beta oxidation of fatty acids with an odd number of methylene groups produces propionyl CoA, which is then converted into succinyl-CoA and fed into the citric acid cycle.[17]
The total energy gained from the complete breakdown of one molecule of glucose by glycolysis, the citric acid cycle, and oxidative phosphorylation equals about 30 ATP molecules, in eukaryotes. The citric acid cycle is called an amphibolic pathway because it participates in both catabolism and anabolism.
Interactive pathway map
Click on genes, proteins and metabolites below to link to respective articles.[18]
Citric_acid_cycle edit
See also
References
- ^ Lowenstein JM (1969). Methods in Enzymology, Volume 13: Citric Acid Cycle. Boston: Academic Press. ISBN 0-12-181870-5.
- ^ Krebs HA, Weitzman PDJ (1987). Krebs' citric acid cycle: half a century and still turning. London: Biochemical Society. ISBN 0-904498-22-0.
- ^ Lane, Nick (2009). Life Ascending: The Ten Great Inventions of Evolution. New York: W.W. Norton & Co. ISBN 0-393-06596-0.
- ^ "The Nobel Prize in Physiology or Medicine 1937". The Nobel Foundation. http://nobelprize.org/nobel_prizes/medicine/laureates/1937/. Retrieved 2011-10-26.
- ^ "The Nobel Prize in Physiology or Medicine 1953". The Nobel Foundation. http://nobelprize.org/nobel_prizes/medicine/laureates/1953/. Retrieved 2011-10-26.
- ^ Gest H (1987). "Evolutionary roots of the citric acid cycle in prokaryotes". Biochem. Soc. Symp. 54: 3–16. PMID 3332996.
- ^ Meléndez-Hevia E, Waddell TG, Cascante M (September 1996). "The puzzle of the Krebs citric acid cycle: assembling the pieces of chemically feasible reactions, and opportunism in the design of metabolic pathways during evolution". J. Mol. Evol. 43 (3): 293–303. PMID 8703096.
- ^ Wolfe RR, Jahoor F (February 1990). "Recovery of labeled CO2 during the infusion of C-1- vs C-2-labeled acetate: implications for tracer studies of substrate oxidation". Am. J. Clin. Nutr. 51 (2): 248–52. PMID 2106256.
- ^ a b c d Stryer L, Berg J, Tymoczko JL (2002). Biochemistry. San Francisco: W.H. Freeman. ISBN 0-7167-4684-0.
- ^ a b Jones RC, Buchanan BB, Gruissem W (2000). Biochemistry & molecular biology of plants (1st ed.). Rockville, Md: American Society of Plant Physiologists. ISBN 0-943088-39-9.
- ^ Johnson JD, Mehus JG, Tews K, Milavetz BI, Lambeth DO (October 1998). "Genetic evidence for the expression of ATP- and GTP-specific succinyl-CoA synthetases in multicellular eucaryotes". J. Biol. Chem. 273 (42): 27580–6. doi:10.1074/jbc.273.42.27580. PMID 9765291.
- ^ Barnes SJ, Weitzman PD (June 1986). "Organization of citric acid cycle enzymes into a multienzyme cluster". FEBS Lett. 201 (2): 267–70. doi:10.1016/0014-5793(86)80621-4. PMID 3086126.
- ^ Rich PR (December 2003). "The molecular machinery of Keilin's respiratory chain". Biochem. Soc. Trans. 31 (Pt 6): 1095–105. doi:10.1042/BST0311095. PMID 14641005.
- ^ Voet D, Voet JG (2004). Biochemistry (3rd ed.). New York: John Wiley & Sons, Inc.. p. 615.
- ^ Denton RM, Randle PJ, Bridges BJ, Cooper RH, Kerbey AL, Pask HT, Severson DL, Stansbie D, Whitehouse S (October 1975). "Regulation of mammalian pyruvate dehydrogenase". Mol. Cell. Biochem. 9 (1): 27–53. doi:10.1007/BF01731731. PMID 171557.
- ^ Koivunen P, Hirsilä M, Remes AM, Hassinen IE, Kivirikko KI, Myllyharju J (February 2007). "Inhibition of hypoxia-inducible factor (HIF) hydroxylases by citric acid cycle intermediates: possible links between cell metabolism and stabilization of HIF". J. Biol. Chem. 282 (7): 4524–32. doi:10.1074/jbc.M610415200. PMID 17182618.
- ^ Halarnkar PP, Blomquist GJ (1989). "Comparative aspects of propionate metabolism". Comp. Biochem. Physiol., B 92 (2): 227–31. doi:10.1016/0305-0491(89)90270-8. PMID 2647392.
- ^ The interactive pathway map can be edited at WikiPathways: "TCACycle_WP78". http://www.wikipathways.org/index.php/Pathway:WP78.
External links
- Citric acid cycle Animation(flash required)
- An animation of the citric acid cycle at Smith College
- Notes on citric acid cycle at rahulgladwin.com
- Citric acid cycle variants at MetaCyc
- Pathways connected to the citric acid cycle at Kyoto Encyclopedia of Genes and Genomes
- A more detailed tutorial animation at johnkyrk.com
- A citric-acid cycle self quiz flash applet at University of Pittsburgh
- The chemical logic behind the citric acid cycle
- The Krebs cycle song by Lynda Jones.
Metabolism (Catabolism, Anabolism) General Cellular respiration Aerobic RespirationGlycolysis → Pyruvate Decarboxylation → Citric Acid Cycle → Oxidative Phosphorylation (Electron Transport Chain + ATP synthase)Specific paths HumanNonhumanOtherNucleotide metabolismOtherbiochemical families: prot · nucl · carb (glpr, alco, glys) · lipd (fata/i, phld, strd, gllp, eico) · amac/i · ncbs/i · ttpy/iCitric Acid Cycle Metabolic Pathway Oxaloacetate Malate Fumarate Succinate Succinyl-CoA 

Acetyl-CoA NADH + H+ NAD+ H2O FADH2 FAD CoA + ATP(GTP) Pi + ADP(GDP) 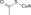
+ H2O 
NADH + H+ + CO2 CoA NAD+ 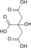
H2O 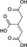
H2O 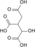
NAD(P)+ NAD(P)H + H+ 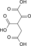
CO2 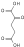
Citrate cis-Aconitate Isocitrate Oxalosuccinate α-Ketoglutarate Metabolism: Citric acid cycle enzymes Cycle Anaplerotic to acetyl-CoAPyruvate dehydrogenase complex (E1, E2, E3)
(regulated by Pyruvate dehydrogenase kinase and Pyruvate dehydrogenase phosphatase)to succinyl-CoAto oxaloacetateMitochondrial
electron transport chain/
oxidative phosphorylationPrimaryComplex I/NADH dehydrogenase · Complex II/Succinate dehydrogenase · Coenzyme Q · Complex III/Coenzyme Q - cytochrome c reductase · Cytochrome c · Complex IV/Cytochrome c oxidase
Coenzyme Q10 synthesis: COQ2 · COQ3 · COQ4 · COQ5 · COQ6 · COQ7 · COQ9 · COQ10A · COQ10B · PDSS1 · PDSS2OtherCategories:- Citric acid cycle
- Biochemistry
- Cellular respiration
- Exercise physiology
- Metabolic pathways
Wikimedia Foundation. 2010.