- Acetate
-
An acetate (pronounced /ˈæsɪteɪt/) is a derivative of acetic acid. This term includes salts and esters, as well as the anion found in solution. Most of the approximately 5 billion kilograms of acetic acid produced annually in industry are used in the production of acetates, which usually take the form of polymers. In nature, acetate is the most common building block for biosynthesis. For example, the fatty acids are produced by connecting C2 units derived from acetate.[1]
Contents
Nomenclature and presentation of formula
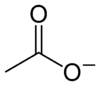
When part of a salt, the formula of the acetate anion is written as CH3CO2−, C2H3O2−, and CH3COO−. Chemists abbreviate acetate as OAc− and AcO−. Thus, HOAc is the abbreviation for acetic acid, NaOAc for sodium acetate, and EtOAc for ethyl acetate.[2][3]
The IUPAC systematic name for acetate is ethanoate. Acetate is an accepted common name.
Salts
The acetate anion, [CH3COO]−, is one of the carboxylate family. It is the conjugate base of acetic acid. Above pH of 5.5, acetic acid converts to acetate:[2]
- CH3COOH ⇌ CH3COO− + H+
Many acetate salts are ionic, indicated by their tendency to dissolve well in water. A commonly encountered acetate in the home is sodium acetate, a white solid that can be prepared by combining vinegar and sodium bicarbonate ("bicarb"):
- CH3COOH + NaHCO3 → CH3COO−Na+ + H2O + CO2
More specialized metal acetates can have complicated structures. Acetate is a relatively strong ligand in coordination chemistry. Examples of acetate complexes include chromium(II) acetate and basic zinc acetate.
Applications
Commercially important acetate salts are aluminium acetate, used in dyeing, ammonium acetate, a precursor to acetamide, and potassium acetate, used as a diuretic. All three salts are colourless and highly soluble in water.[4]
Esters
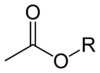
Acetate esters have the general formula CH3CO2R, where R is an organyl group. The esters are the dominant forms of acetate in the marketplace. Unlike the acetate salts, acetate esters are often liquids, lipophilic, and sometimes volatile. They are popular because they have inoffensive, often sweet odors, they are inexpensive, and they are usually of low toxicity.
Applications
Almost half of acetic acid production is consumed in the production of vinyl acetate, precursor to polyvinyl alcohol, which is a component of many paints. The second largest use of acetic acid is consumed in the production of cellulose acetate. In fact, "acetate" is jargon for cellulose acetate, which is used in the production of fibres or diverse products, e.g. the acetate discs used in audio record production. Cellulose acetate can be found in many household products. Many industrial solvents are acetates, including methyl acetate, ethyl acetate, isopropyl acetate, ethylhexyl acetate. Butyl acetate is a fragrance used in food products.[4]
Acetate in biology
Acetate is a common anion in biology. It is mainly utilized by organisms in the form of acetyl coenzyme A.[5]
Intraperitoneal injection of sodium acetate (20 or 60 mg per kg body mass) was found to induce headache in sensitized rats, and it has been proposed that acetate resulting from oxidation of ethanol is a major factor in causing hangovers. Increased serum acetate levels lead to accumulation of adenosine in many tissues including the brain, and administration of the adenosine receptor antagonist caffeine to rats after ethanol was found to decrease nociceptive behavior.[6][7]
References
- ^ March, J. “Advanced Organic Chemistry” 4th Ed. J. Wiley and Sons, 1992: New York. ISBN 0-471-60180-2.
- ^ a b Zumdahl, S. S. “Chemistry” Heath, 1986: Lexington, MA. ISBN 0-669-04529-2.
- ^ Prior to the discovery and naming of actinium, the abbreviation "Ac" (or "AC") was sometimes used in chemical formulas to indicate the acetate ion. For example, the formula for sodium acetate might be given as "NaAc", rather than the more modern "NaCH3COO" or "NaC2H3O2".
- ^ a b Hosea Cheung, Robin S. Tanke, G. Paul Torrence "Acetic acid" in Ullmann's Encyclopedia of Industrial Chemistry Weinheim, Germany: Wiley-VCH, 2005. doi:10.1002/14356007.a01 045
- ^ Nelson, D. L.; Cox, M. M. "Lehninger, Principles of Biochemistry" 3rd Ed. Worth Publishing: New York, 2000. ISBN 1-57259-153-6.
- ^ 'Acetate Causes Alcohol Hangover Headache in Rats' by Christina Maxwell et al., PLoS ONE 5(12): e15963.
- ^ 'Is coffee the real cure for a hangover?' by Bob Holmes, New Scientist, Jan. 15 2011, p. 17.
Structures
-
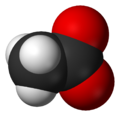
space-filling model of the acetate anion -
ball-and-stick model of the acetate anion -
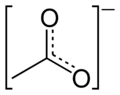
resonance hybrid of the acetate anion -
canonical forms of the acetate anion
See also
Categories:- Acetates
- Carboxylate anions
Wikimedia Foundation. 2010.