- Methyl acetate
-
Not to be confused with menthyl acetate.
Methyl acetate[1] 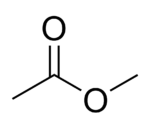
 Methyl acetateSystematic nameMethyl ethanoate
Methyl acetateSystematic nameMethyl ethanoateIdentifiers CAS number 79-20-9 
PubChem 6584 ChemSpider 6335 
UNII W684QT396F 
KEGG C17530 
ChEMBL CHEMBL14079 
Jmol-3D images Image 1 - O=C(OC)C
Properties Molecular formula C3H6O2 Molar mass 74.08 g mol−1 Density 0.932 g cm−3 Melting point -98 °C, 175 K, -144 °F
Boiling point 56.9 °C, 330 K, 134 °F
Refractive index (nD) 1.361 Hazards MSDS External MSDS NFPA 704 Flash point -9 °C Related compounds Related esters Methyl formate
Ethyl acetate acetate (verify) (what is:
acetate (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Methyl acetate, also known as MeOAc, acetic acid methyl ester or methyl ethanoate, is a carboxylate ester with the formula CH3COOCH3. It is normally a flammable liquid with a characteristic, pleasant smell like certain glues or nail polish removers. Methyl acetate has characteristics very similar to its analog ethyl acetate. Methyl acetate is used as a solvent, being weakly polar and lipophilic. Methyl acetate has a solubility of 25% in water at room temperature. At elevated temperature its solubility in water is much higher. Methyl acetate is not stable in the presence of strong aqueous bases or acids. Methyl acetate is VOC exempt.[2]
Contents
Preparation and reactions
Methyl acetate is produced industrially via the carbonylation of methanol as a byproduct of the production of acetic acid.[3] Although not of commercial significance, methyl acetate arises by esterification in the presence of strong acids such as sulfuric acid.
Reactions
In the presence of strong bases such as sodium hydroxide or strong acids such as hydrochloric acid or sulfuric acid it is hydrolyzed back into methanol and acetic acid, especially at elevated temperature. The conversion of methyl acetate back into its components, by an acid, is a first-order reaction with respect to the ester. The reaction of methyl acetate and a base, for example sodium hydroxide, is a second-order reaction with respect to both reactants.
Applications
A major use of methyl acetate is as a volatile low toxicity solvent in glues, paints, and nail polish removers.
Acetic anhydride is produced by carbonylation of methyl acetate in a process that was inspired by the Monsanto acetic acid synthesis.[4]
References
- ^ Merck Index, 12th Edition, 6089.
- ^ Zeno, W. Wicks, JR, Frank N. Jones, S. Peter Pappas, and Douglas A. Wicks. Organic Coatings Hoboken, New Jersey: Wiley, 2007. ISBN# 978-0-471-69806-7
- ^ Hosea Cheung, Robin S. Tanke, G. Paul Torrence “Acetic Acid” in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a01_045
- ^ Zoeller, J. R.; Agreda, V. H.; Cook, S. L.; Lafferty, N. L.; Polichnowski, S. W.; Pond, D. M. (1992). "Eastman Chemical Company Acetic Anhydride Process". Catalysis Today 13: 73–91. doi:10.1016/0920-5861(92)80188-S.
Categories:- Methyl esters
- Ester solvents
- Acetate esters
Wikimedia Foundation. 2010.

