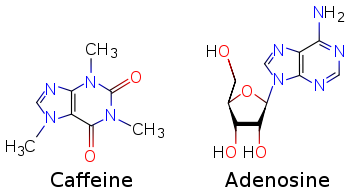
- Adenosine
-
Adenosine 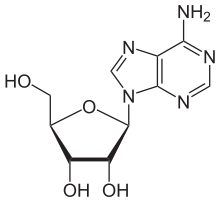
Systematic (IUPAC) name (2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-(hydroxymethyl)oxolane-3,4-diol Clinical data Trade names Adenocard AHFS/Drugs.com monograph Pregnancy cat. C Legal status Australia - Legal; UK - Legal; US - Rx only Routes Intravenous Pharmacokinetic data Bioavailability Rapidly cleared from circulation via cellular uptake Protein binding No Metabolism Rapidly converted to inosine and adenosine monophosphate Half-life cleared plasma <30 seconds - half life <10 seconds Excretion can leave cell intact or can be degraded to hypoxanthine, xanthine, and ultimately uric acid Identifiers CAS number 58-61-7 
ATC code C01EB10 PubChem CID 60961 DrugBank DB00640 ChemSpider 54923 
UNII K72T3FS567 
KEGG C00212 
ChEBI CHEBI:16335 
ChEMBL CHEMBL606298 
Chemical data Formula C10H13N5O4 Mol. mass 267.241 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Adenosine is a purine nucleoside comprising a molecule of adenine attached to a ribose sugar molecule (ribofuranose) moiety via a β-N9-glycosidic bond.
Adenosine plays an important role in biochemical processes, such as energy transfer—as adenosine triphosphate (ATP) and adenosine diphosphate (ADP)—as well as in signal transduction as cyclic adenosine monophosphate, cAMP. It is also an inhibitory neurotransmitter, believed to play a role in promoting sleep and suppressing arousal, with levels increasing with each hour an organism is awake.
Adenosine is often abbreviated Ado.
Contents
Pharmacological effects
Adenosine is an endogenous purine nucleotide that modulates many physiological processes. Cellular signaling by adenosine occurs through four known adenosine receptor subtypes (A1, A2A, A2B, and A3).[1]
Extracellular adenosine concentrations from normal cells are approximately 300 nM; however, in response to cellular damage (e.g. in inflammatory or ischemic tissue), these concentrations are quickly elevated (600–1,200 nM). Thus, in regard to stress or injury, the function of adenosine is primarily that of cytoprotection preventing tissue damage during instances of hypoxia, ischemia, and seizure activity. Activation of A2A receptors produces a constellation of responses that in general can be classified as anti-inflammatory.[citation needed]
Adenosine receptors
Main article: Adenosine receptorThe different adenosine receptor subtypes (A1, A2A, A2B, and A3) are all seven transmembrane spanning G-protein coupled receptors. These four receptor subtypes are further classified based on their ability to either stimulate or inhibit adenylate cyclase activity. The A2A and A2B receptors couple to Gs and mediate the stimulation of adenylate cyclase, while the A1 and A3 adenosine receptors couple to Gi which inhibits adenylate cyclase activity. Additionally, A1 receptors couple to Go, which has been reported to mediate adenosine inhibition of Ca2+ conductance, whereas A2B and A3 receptors also couple to Gq and stimulate phospholipase activity.
Anti-inflammatory properties
Adenosine is believed to be an anti-inflammatory agent at the A(2A) receptor.[2][3] Topical treatment of adenosine to foot wounds in diabetes mellitus has been shown in lab animals to drastically increase tissue repair and reconstruction. Topical administration of adenosine for use in wound healing deficiencies and diabetes mellitus in humans is currently under clinical investigation.
Methotrexate's anti-inflammatory effect may be due to its stimulation of adenosine release.
Action on the heart
When administered intravenously, adenosine causes transient heart block in the Atrioventricular (AV) node. This is mediated via the A1 receptor, inhibiting adenylyl cyclase, reducing cAMP and so causing cell hyperpolarization by increasing outward K+ flux. It also causes endothelial dependent relaxation of smooth muscle as is found inside the artery walls. This causes dilation of the "normal" segments of arteries; i.e. where the endothelium is not separated from the tunica media by atherosclerotic plaque. This feature allows physicians to use adenosine to test for blockages in the coronary arteries, by exaggerating the difference between the normal and abnormal segments.
In individuals suspected of suffering from a supraventricular tachycardia (SVT), adenosine is used to help identify the rhythm. Certain SVTs can be successfully terminated with adenosine.[4] This includes any re-entrant arrhythmias that require the AV node for the re-entry (e.g., AV reentrant tachycardia (AVRT), AV nodal reentrant tachycardia (AVNRT). In addition, atrial tachycardia can sometimes be terminated with adenosine.
Adenosine has an indirect effect on atrial tissue causing a shortening of the refractory period. When administered via a central lumen catheter, adenosine has been shown to initiate atrial fibrillation because of its effect on atrial tissue. In individuals with accessory pathways, the onset of atrial fibrillation can lead to a life-threatening ventricular fibrillation.
Fast rhythms of the heart that are confined to the atria (e.g., atrial fibrillation, atrial flutter) or ventricles (e.g., monomorphic ventricular tachycardia) and do not involve the AV node as part of the re-entrant circuit are not typically converted by adenosine. However, the ventricular response rate is temporarily slowed with adenosine in such cases.
Because of the effects of adenosine on AV node-dependent SVTs, adenosine is considered a class IV antiarrhythmic agent. When adenosine is used to cardiovert an abnormal rhythm, it is normal for the heart to enter ventricular asystole for a few seconds. This can be disconcerting to a normally conscious patient, and is associated with angina-like sensations in the chest.[5]
By nature of caffeine's purine structure[6] it binds to some of the same receptors as adenosine.[6] The pharmacological effects of adenosine may therefore be blunted in individuals who are taking large quantities of methylxanthines (e.g., caffeine, found in coffee and tea, or theobromine, as found in chocolate). [7][citation needed]
Action in the central nervous system
Generalized, adenosine has an inhibitory effect in the central nervous system (CNS). Caffeine's stimulatory effects, on the other hand, are primarily (although not entirely) credited to its inhibition of adenosine by binding to the same receptors, and therefore effectively blocking adenosine receptors in the CNS. This reduction in adenosine activity leads to increased activity of the neurotransmitters dopamine and glutamate.
Dosage
When given for the evaluation or treatment of a supraventricular tachycardia (SVT), the initial dose is 6 mg, given as a rapid parenteral infusion. Due to adenosine's extremely short half-life, the IV line is started as proximal (near) to the heart as possible, such as the cubital fossa. The IV push is often followed with an immediate flush of 10-20ccs of saline. If this has no effect (i.e. no evidence of transient AV block), a 12 mg dose can be given 1–2 minutes after the first dose. AHA 2010 IS NO LONGER RECOMMENDING A SECOND DOSE OF 12MG. PLEASE VIEW ALGORITHM AS CONFIRMATION. Some clinicians may prefer to administer a higher dose (typically 18 mg), rather than repeat a dose that apparently had no effect. When given to dilate the arteries, such as in a "stress test", the dosage is typically 0.14 mg/kg/min, administered for 4 or 6 minutes, depending on the protocol.
The recommended dose may be increased in patients on theophylline since methylxanthines prevent binding of adenosine at receptor sites. The dose is often decreased in patients on dipyridamole (Persantine) and diazepam (Valium) because adenosine potentiates the effects of these drugs. The recommended dose is also reduced by half in patients who are presenting congestive heart failure, myocardial infarction, shock, hypoxia, and/or hepatic or renal insufficiency, and in elderly patients.
Drug interactions
Dopamine may precipitate toxicity in the patient. Carbamazepine may increase heart block. Theophylline and caffeine (methylxanthines) competitively antagonize adenosine's effects; an increased dose of adenosine may be required. Dipyridamole potentiates the action of adenosine, requiring the use of lower doses.
Contraindications
Common Contraindications for adenosine are:
- 2nd or 3rd degree heart block (without a pacemaker)
- Sick sinus syndrome (without a pacemaker)
- Long QT syndrome
- Severe hypotension
- Decompensated heart failure
- Asthma, traditionally an absolute CI this is being contended and it is now considered relative (however, selective adenosine agonists are being investigated for use in treatment of asthma)[8]
- Poison/drug-induced tachycardia
In Wolff-Parkinson-White syndrome, adenosine may be administered if equipment for cardioversion is immediately available as a backup.
Side effects
Many individuals experience facial flushing, a temporary rash on the chest, lightheadedness, diaphoresis, or nausea after administration of adenosine due to its vasodilatory effects. Metallic taste is a hallmark side effect of adenosine administration. These symptoms are transitory, usually lasting less than one minute. It is classically associated with a sense of "impending doom", more prosaically described as apprehension. This lasts a few seconds after administration of a bolus dose, during transient asystole induced by intravenous administration. In some cases adenosine can make patients' limbs feel numb for about 2–5 minutes after administration intravenously depending on the dosage (usually above 12 mg).
Metabolism
Adenosine used as a second messenger can be the result of de novo purine biosynthesis via adenosine monophosphate (AMP), though it is possible other pathways exist.[9]
When adenosine enters the circulation, it is broken down by adenosine deaminase, which is present in red cells and the vessel wall.
Dipyridamole, an inhibitor of adenosine deaminase, allows adenosine to accumulate in the blood stream. This causes an increase in coronary vasodilatation.
Adenosine deaminase deficiency is a known cause of immunodeficiency.
Analogs and viruses
The adenosine analog, NITD008 has been reported to directly inhibit the recombinant an RNA-dependent RNA polymerase of the dengue virus by terminating its RNA chain synthesis. This suppresses peak viremia, rise in cytokines and prevented infected animal from death raising the possibility of a new treatment for this flavivirus.[10] The 7-deaza-adenosine analog has been shown to inhibit the replication of the hepatitis C virus.[11] Such adenosine analogs are potentially clinically useful since they can be taken orally.
See also
- Adenosine receptors
- Health effects of caffeine
References
- ^ Haskó G, Linden J, Cronstein B, Pacher P (September 2008). "Adenosine receptors: therapeutic aspects for inflammatory and immune diseases". Nat Rev Drug Discov 7 (9): 759–70. doi:10.1038/nrd2638. PMC 2568887. PMID 18758473. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2568887.
- ^ Nakav S, Chaimovitz C, Sufaro Y (2008). Bozza, Patricia. ed. "Anti-Inflammatory Preconditioning by Agonists of Adenosine A1 Receptor". PLoS ONE 3 (5): e2107. doi:10.1371/journal.pone.0002107. PMC 2329854. PMID 18461129. http://www.plosone.org/article/info:doi/10.1371/journal.pone.0002107.
- ^ Trevethick MA, Mantell SJ, Stuart EF, Barnard A, Wright KN, Yeadon M (October 2008). "Treating lung inflammation with agonists of the adenosine A2A receptor: promises, problems and potential solutions". Br. J. Pharmacol. 155 (4): 463–74. doi:10.1038/bjp.2008.329. PMC 2579671. PMID 18846036. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2579671.
- ^ Mitchell J, Lazarenko G (November 2008). "Wide QRS complex tachycardia. Diagnosis: Supraventricular tachycardia with aberrant conduction; intravenous (IV) adenosine". CJEM 10 (6): 572–3, 581. PMID 19000353. http://caep.ca/template.asp?id=563f5c92af544619b2ac53fbba2ce6c5.
- ^ Pijls, Nico H. J.; Bernard De Bruyne (2000). Coronary Pressure. Springer. ISBN 0-7923-6170-9.
- ^ a b "Caffeine". Chemistry Explained. http://www.chemistryexplained.com/Bo-Ce/Caffeine.html.
- ^ "Vitamin B4". R&S Pharmchem. April 2011. http://www.rspharmchem.com/vitamin-b4.htm.
- ^ Brown RA, Spina D, Page CP (March 2008). "Adenosine receptors and asthma". Br. J. Pharmacol. 153 Suppl 1 (S1): S446–56. doi:10.1038/bjp.2008.22. PMC 2268070. PMID 18311158. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2268070.
- ^ PMID 8020339
- ^ Yin Z, Chen YL, Schul W, Wang QY, Gu F, Duraiswamy J, Reddy Kondreddi R, Niyomrattanakit P, Lakshminarayana SB, Goh A, Xu HY, Liu W, Liu B, Lim JY, Ng CY, Qing M, Lim CC, Yip A, Wang G, Chan WL, Tan HP, Lin K, Zhang B, Zou G, Bernard KA, Garrett C, Beltz K, Dong M, Weaver M, He H, Pichota A, Dartois V, Keller TH, Shi PY. (2009). Proc Natl Acad Sci U S A. 106: 20435–20439 doi:10.1073/pnas.0907010106 PMID 19918064
- ^ Olsen, DB; Eldrup, AB; Bartholomew, L; Bhat, B; Bosserman, MR; Ceccacci, A; Colwell, LF; Fay, JF et al. (2004). "A 7-Deaza-Adenosine Analog Is a Potent and Selective Inhibitor of Hepatitis C Virus Replication with Excellent Pharmacokinetic Properties". Antimicrobial agents and chemotherapy 48 (10): 3944–53. doi:10.1128/AAC.48.10.3944-3953.2004. PMC 521892. PMID 15388457. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=521892.
External links
Nucleic acid constituents Nucleobase Nucleoside Nucleotide
(Nucleoside monophosphate)Nucleoside diphosphate Nucleoside triphosphate biochemical families: prot · nucl · carb (glpr, alco, glys) · lipd (fata/i, phld, strd, gllp, eico) · amac/i · ncbs/i · ttpy/iAntiarrhythmic agents (C01B) Channel blockers class Ib (Phase 3←)class Ic (Phase 0→)Amiodarone • Dronedarone • Bretylium • Bunaftine • Dofetilide • Ibutilide • Nifekalant • Sotalol • Tedisamil • Vernakalant • E-4031Receptor agonists
and antagonistsAdenosineIon transporters Adenosinergics Receptor ligands 2-(1-Hexynyl)-N-methyladenosine • 2-Cl-IB-MECA • 2'-MeCCPA • 5'-N-ethylcarboxamidoadenosine • ATL-146e • BAY 60–6583 • CCPA • CGS-21680 • CP-532,903 • GR 79236 • LUF-5835 • LUF-5845 • N6-Cyclopentyladenosine • Regadenoson • SDZ WAG 994 • UK-432,0978-Phenyl-1,3-dipropylxanthine • Acefylline • Aminophylline • Bamifylline • Caffeine • CGS-15943 • 8-Chlorotheophylline • CPX • CVT-6883 • Dimethazan • DPCPX • Fenethylline • Istradefylline • KF-26777 • MRE3008F20 • MRS-1220 • MRS-1334 • MRS-1706 • MRS-1754 • MRS-3777 • Paraxanthine • Pentoxifylline • Preladenant • Propentofylline • PSB-10 • PSB-11 • PSB 36 • PSB-603 • PSB-788 • PSB-1115 • Rolofylline • SCH-442,416 • SCH-58261 • Theobromine • Theophylline • VUF-5574 • ZM-241,385Reuptake inhibitors ENT inhibitorsVNT inhibitorsNeurotransmitters Amino acids Alanine · Aspartate · Cycloserine · DMG · GABA · Glutamate · Glycine · Hypotaurine · Kynurenic acid (Transtorine) · NAAG (Spaglumic acid) · NMG (Sarcosine) · Serine · Taurine · TMG (Betaine)
Endocannabinoids 2-AG · 2-AGE (Noladin ether) · AEA (Anandamide) · NADA · OAE (Virodhamine) · Oleamide · PEA (Palmitoylethanolamide) · RVD-Hpα · Hp (Hemopressin)
Gasotransmitters Monoamines Purines Trace amines 3-ITA · 5-MeO-DMT · Bufotenin · DMT · NMT · Octopamine · Phenethylamine · Synephrine · Thyronamine · Tryptamine · Tyramine
Others 1,4-BD · Acetylcholine · GBL · GHB · Histamine
See also Template:NeuropeptidesCategories:- Antiarrhythmic agents
- Drugs acting on the cardiovascular system
- Vasodilators
- Nucleosides
- Purines
- Ribosides
Wikimedia Foundation. 2010.