- Aspartic acid
-
Aspartic acid 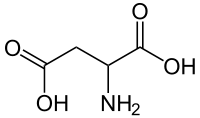
 Trivial: Aspartic acid
Trivial: Aspartic acid
Systematic: 2-Aminobutanedioic acidOther namesAminosuccinic acid, asparagic acid, asparaginic acid[1]Identifiers CAS number 617-45-8  ,
,
56-84-8 (L-isomer)
1783-96-6 (D-isomer)PubChem 424 ChemSpider 411 
UNII 28XF4669EP 
EC-number 200-291-6 KEGG C16433 
ChEBI CHEBI:22660 
ChEMBL CHEMBL139661 
Jmol-3D images Image 1
Image 2- O=C(O)CC(N)C(=O)O
C(C(C(=O)O)N)C(=O)O
Properties Molecular formula C4H7NO4 Molar mass 133.1 g mol−1 Hazards MSDS External MSDS EU Index not listed Supplementary data page Structure and
propertiesn, εr, etc. Thermodynamic
dataPhase behaviour
Solid, liquid, gasSpectral data UV, IR, NMR, MS  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Aspartic acid (abbreviated as Asp or D; Asx or B represent either aspartic acid or asparagine)[2] is an α-amino acid with the chemical formula HOOCCH(NH2)CH2COOH. The carboxylate anion, salt, or ester of aspartic acid is known as aspartate. The L-isomer of aspartate is one of the 20 proteinogenic amino acids, i.e., the building blocks of proteins. Its codons are GAU and GAC.
Aspartic acid is, together with glutamic acid, classified as an acidic amino acid with a pKa of 4.0. Aspartate is pervasive in biosynthesis. As with all amino acids, the presence of acid protons depends on the residue's local chemical environment and the pH of the solution.
Contents
Discovery
Aspartic acid was first discovered in 1827 by Plisson, synthesized by boiling asparagine (which had been isolated from asparagus juice in 1806) with a base.[3]
Forms and nomenclature
The term "aspartic acid" refers to either of two forms or a mixture of two.[2] Of these two forms, only one, "L-aspartic acid", is directly incorporated into amino acids. The biological roles of its counterpart, "D-aspartic acid" are more limited. Where enzymatic synthesis will produce one or the other, most chemical syntheses will produce both forms, "DL-aspartic acid," known as a racemic mixture.
Role in biosynthesis of amino acids
Aspartate is non-essential in mammals, being produced from oxaloacetate by transamination. It can also be made in the Urea Cycle from Ornithine and Citrulline. In plants and microorganisms, aspartate is the precursor to several amino acids, including four that are essential for humans: methionine, threonine, isoleucine, and lysine. The conversion of aspartate to these other amino acids begins with reduction of aspartate to its "semialdehyde," O2CCH(NH2)CH2CHO.[4] Asparagine is derived from aspartate via transamidation:
- -O2CCH(NH2)CH2CO2- + GC(O)NH3+ O2CCH(NH2)CH2CONH3+ + GC(O)O
(where GC(O)NH2 and GC(O)OH are glutamine and glutamic acid, respectively)
Other biochemical roles
Aspartate is also a metabolite in the urea cycle and participates in gluconeogenesis. It carries reducing equivalents in the malate-aspartate shuttle, which utilizes the ready interconversion of aspartate and oxaloacetate, which is the oxidized (dehydrogenated) derivative of malic acid. Aspartate donates one nitrogen atom in the biosynthesis of inosine, the precursor to the purine bases.
Neurotransmitter
Aspartate (the conjugate base of aspartic acid) stimulates NMDA receptors, though not as strongly as the amino acid neurotransmitter glutamate does.[5]
Sources
Dietary sources
Aspartic acid is not an essential amino acid, which means that it can be synthesized from central metabolic pathway intermediates in humans. Aspartic acid is found in:
- Animal sources: luncheon meats, sausage meat, wild game
- Vegetable sources: sprouting seeds, oat flakes, avocado, asparagus[citation needed], young sugarcane, and molasses from sugar beets.[1]
- Dietary supplements, either as aspartic acid itself or salts (such as magnesium aspartate)
- The sweetener aspartame (NutraSweet, Equal, Canderel, etc.)
Chemical synthesis
Racemic aspartic acid can be synthesized from diethyl sodium phthalimidomalonate, (C6H4(CO)2NC(CO2Et)2).[6]
The major disadvantage of the above technique is that equimolar amounts of each enantiomer are made, the body only utilises L-amino acids. Using biotechnology it is now possible to use immobilised enzymes to create just one type of enantiomer owing to their stereospecificity. Aspartic acid is made synthetically using ammonium fumarate and aspartase from E.coli, E.coli usually breaks down the aspartic acid as a nitrogen source but using excess amounts of ammonium fumarate a reversal of the enzyme's job is possible, and so aspartic acid is made to very high yields, 98.7 mM from 1 M.
References
- ^ a b "862. Aspartic acid". The Merck Index (11th ed.). 1989. p. 132. ISBN 091191028X.
- ^ a b "Nomenclature and symbolism for amino acids and peptides (IUPAC-IUB Recommendations 1983)", Pure Appl. Chem. 56 (5): 595–624, 1984, doi:10.1351/pac198456050595.
- ^ R.H.A. Plimmer (1912) [1908]. R.H.A. Plimmer & F.G. Hopkins. ed. The chemical composition of the proteins. Monographs on biochemistry. Part I. Analysis (2nd ed.). London: Longmans, Green and Co.. p. 112. http://books.google.com/?id=7JM8AAAAIAAJ&pg=PA112. Retrieved January 18, 2010.
- ^ Lehninger, Albert L.; Nelson, David L.; Cox, Michael M. (2000), Principles of Biochemistry (3rd ed.), New York: W. H. Freeman, ISBN 1-57259-153-6.
- ^ Chen, Philip E.; Geballe, Matthew T.; Stansfeld, Phillip J.; Johnston, Alexander R.; Yuan, Hongjie; Jacob, Amanda L.; Snyder, James P.; Traynelis, Stephen F. et al. (2005). "Structural Features of the Glutamate Binding Site in Recombinant NR1/NR2A N-Methyl-D-aspartate Receptors Determined by Site-Directed Mutagenesis and Molecular Modeling". Mol. Pharmacol. 67 (5): 1470–84. doi:10.1124/mol.104.008185. PMID 15703381. http://molpharm.aspetjournals.org/cgi/content/full/67/5/1470.
- ^ Dunn, M. S.; Smart, B. W. (1950), "DL-Aspartic Acid", Org. Synth. 30: 7, http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=CV4P0055; Coll. Vol. 4: 55.
</ref>
See also
- Aspartate transaminase
- Sodium poly(aspartate), a synthetic polyamide
- American Chemical Society (21 April 2010). "Ancestral Eve' Crystal May Explain Origin of Life's Left-Handedness". ScienceDaily. http://www.sciencedaily.com/releases/2010/04/100421121501.htm. Retrieved 2010-04-21.
The 20 common amino acids By properties AliphaticBranched-chain amino acids (Valine · Isoleucine · Leucine) · Methionine · Alanine · Proline · GlycineAromaticPolar, unchargedPositive charge (pKa)Negative charge (pKa)GeneralOther classifications biochemical families: prot · nucl · carb (glpr, alco, glys) · lipd (fata/i, phld, strd, gllp, eico) · amac/i · ncbs/i · ttpy/i Neurotransmitters Amino acids Alanine · Aspartate · Cycloserine · DMG · GABA · Glutamate · Glycine · Hypotaurine · Kynurenic acid (Transtorine) · NAAG (Spaglumic acid) · NMG (Sarcosine) · Serine · Taurine · TMG (Betaine)
Endocannabinoids 2-AG · 2-AGE (Noladin ether) · AEA (Anandamide) · NADA · OAE (Virodhamine) · Oleamide · PEA (Palmitoylethanolamide) · RVD-Hpα · Hp (Hemopressin)
Gasotransmitters Monoamines Purines Trace amines 3-ITA · 5-MeO-DMT · Bufotenin · DMT · NMT · Octopamine · Phenethylamine · Synephrine · Thyronamine · Tryptamine · Tyramine
Others 1,4-BD · Acetylcholine · GBL · GHB · Histamine
See also Template:NeuropeptidesCategories:- Proteinogenic amino acids
- Glucogenic amino acids
- Acidic amino acids
- Dicarboxylic acids
- Neurotransmitters
- Urea cycle
- O=C(O)CC(N)C(=O)O
Wikimedia Foundation. 2010.
