- Glutamic acid
-
Glutamic acid 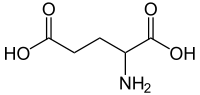
 Glutamic acidOther names2-Aminopentanedioic acid
Glutamic acidOther names2-Aminopentanedioic acid
2-Aminoglutaric acidIdentifiers CAS number 617-65-2  ,
,
56-86-0 (L-isomer)
6893-26-1 (D-isomer)PubChem 611 ChemSpider 591 
UNII 61LJO5I15S 
EC-number 210-522-2 KEGG D04341 
ChEBI CHEBI:18237 
ChEMBL CHEMBL276389 
IUPHAR ligand 1369 Jmol-3D images Image 1 - C(CC(=O)O)C(C(=O)O)N
Properties Molecular formula C5H9NO4 Molar mass 147.13 g mol−1 Appearance white crystalline powder Density 1.4601 (20 °C) Melting point 199 °C decomp.
Solubility in water 8.64 g/l (25 °C) [1] Supplementary data page Structure and
propertiesn, εr, etc. Thermodynamic
dataPhase behaviour
Solid, liquid, gasSpectral data UV, IR, NMR, MS  acid (verify) (what is:
acid (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Glutamic acid (abbreviated as Glu or E) is one of the 20 proteinogenic amino acids, and its codons are GAA and GAG. It is a non-essential amino acid. The carboxylate anions and salts of glutamic acid are known as glutamates. In neuroscience, glutamate is an important neurotransmitter that plays a key role in long-term potentiation and is important for learning and memory.[2]
Contents
Chemistry
The side chain carboxylic acid functional group has pKa of 4.1 and exists in its negatively charged deprotonated carboxylate form at physiological pH ranging from 7.35 to 7.45.
History
Main article: Glutamic acid (flavor)Although they occur naturally in many foods, the flavor contributions made by glutamic acid and other amino acids were only scientifically identified early in the twentieth century. The substance was discovered and identified in the year 1866, by the German chemist Karl Heinrich Leopold Ritthausen. In 1907 Japanese researcher Kikunae Ikeda of the Tokyo Imperial University identified brown crystals left behind after the evaporation of a large amount of kombu broth as glutamic acid. These crystals, when tasted, reproduced the ineffable but undeniable flavor he detected in many foods, most especially in seaweed. Professor Ikeda termed this flavor umami. He then patented a method of mass-producing a crystalline salt of glutamic acid, monosodium glutamate.[3][4]
Biosynthesis
Reactants Products Enzymes Glutamine + H2O → Glu + NH3 GLS, GLS2 NAcGlu + H2O → Glu + Acetate (unknown) α-ketoglutarate + NADPH + NH4+ → Glu + NADP+ + H2O GLUD1, GLUD2[5] α-ketoglutarate + α-amino acid → Glu + α-keto acid transaminase 1-Pyrroline-5-carboxylate + NAD+ + H2O → Glu + NADH ALDH4A1 N-formimino-L-glutamate + FH4 → Glu + 5-formimino-FH4 FTCD Function and uses
Metabolism
Glutamate is a key molecule in cellular metabolism. In humans, dietary proteins are broken down by digestion into amino acids, which serve as metabolic fuel for other functional roles in the body. A key process in amino acid degradation is transamination, in which the amino group of an amino acid is transferred to an α-ketoacid, typically catalysed by a transaminase. The reaction can be generalised as such:
- R1-amino acid + R2-α-ketoacid ⇌ R1-α-ketoacid + R2-amino acid
A very common α-keto acid is α-ketoglutarate, an intermediate in the citric acid cycle. Transamination of α-ketoglutarate gives glutamate. The resulting α-ketoacid product is often a useful one as well, which can contribute as fuel or as a substrate for further metabolism processes. Examples are as follows:
- Alanine + α-ketoglutarate ⇌ pyruvate + glutamate
- Aspartate + α-ketoglutarate ⇌ oxaloacetate + glutamate
Both pyruvate and oxaloacetate are key components of cellular metabolism, contributing as substrates or intermediates in fundamental processes such as glycolysis, gluconeogenesis, and the citric acid cycle.
Glutamate also plays an important role in the body's disposal of excess or waste nitrogen. Glutamate undergoes deamination, an oxidative reaction catalysed by glutamate dehydrogenase[5], as follows:
Ammonia (as ammonium) is then excreted predominantly as urea, synthesised in the liver. Transamination can, thus, be linked to deamination, effectively allowing nitrogen from the amine groups of amino acids to be removed, via glutamate as an intermediate, and finally excreted from the body in the form of urea.
Neurotransmitter
Glutamate is the most abundant excitatory neurotransmitter in the vertebrate nervous system. At chemical synapses, glutamate is stored in vesicles. Nerve impulses trigger release of glutamate from the pre-synaptic cell. In the opposing post-synaptic cell, glutamate receptors, such as the NMDA receptor, bind glutamate and are activated. Because of its role in synaptic plasticity, glutamate is involved in cognitive functions like learning and memory in the brain.[6] The form of plasticity known as long-term potentiation takes place at glutamatergic synapses in the hippocampus, neocortex, and other parts of the brain. Glutamate works not only as a point-to-point transmitter but also through spill-over synaptic crosstalk between synapses in which summation of glutamate released from a neighboring synapse creates extrasynaptic signaling/volume transmission.[7]
Glutamate transporters[8] are found in neuronal and glial membranes. They rapidly remove glutamate from the extracellular space. In brain injury or disease, they can work in reverse, and excess glutamate can accumulate outside cells. This process causes calcium ions to enter cells via NMDA receptor channels, leading to neuronal damage and eventual cell death, and is called excitotoxicity. The mechanisms of cell death include
- Glu/Ca2+-mediated promotion of transcription factors for pro-apoptotic genes, or downregulation of transcription factors for anti-apoptotic genes
Excitotoxicity due to glutamate occurs as part of the ischemic cascade and is associated with stroke[2] and diseases like amyotrophic lateral sclerosis, lathyrism, autism, some forms of mental retardation, and Alzheimer's disease.[2][10]
Glutamic acid has been implicated in epileptic seizures. Microinjection of glutamic acid into neurons produces spontaneous depolarisations around one second apart, and this firing pattern is similar to what is known as paroxysmal depolarizing shift in epileptic attacks. This change in the resting membrane potential at seizure foci could cause spontaneous opening of voltage-activated calcium channels, leading to glutamic acid release and further depolarization.
Experimental techniques to detect glutamate in intact cells include using a genetically-engineered nanosensor.[11] The sensor is a fusion of a glutamate-binding protein and two fluorescent proteins. When glutamate binds, the fluorescence of the sensor under ultraviolet light changes by resonance between the two fluorophores. Introduction of the nanosensor into cells enables optical detection of the glutamate concentration. Synthetic analogs of glutamic acid that can be activated by ultraviolet light and two-photon excitation microscopy have also been described.[12] This method of rapidly uncaging by photostimulation is useful for mapping the connections between neurons, and understanding synapse function.
Evolution of glutamate receptors is entirely the opposite in invertebrates, in particular, arthropods and nematodes, where glutamate stimulates glutamate-gated chloride channels.[citation needed] The beta subunits of the receptor respond with very high affinity to glutamate and glycine.[13] Targeting these receptors has been the therapeutic goal of anthelmintic therapy using avermectins. Avermectins target the alpha-subunit of glutamate-gated chloride channels with high affinity.[14] These receptors have also been described in arthropods, such as Drosophila melanogaster[15] and Lepeophtheirus salmonis.[16] Irreversible activation of these receptors with avermectins results in hyperpolarization at synapses and neuromuscular junctions resulting in flaccid paralysis and death of nematodes and arthropods.
Brain nonsynaptic glutamatergic signaling circuits
Extracellular glutamate in Drosophila brains has been found to regulate postsynaptic glutamate receptor clustering, via a process involving receptor desensitization.[17] A gene expressed in glial cells actively transports glutamate into the extracellular space,[17] while, in the nucleus accumbens-stimulating group II metabotropic glutamate receptors, this gene was found to reduce extracellular glutamate levels.[18] This raises the possibility that this extracellular glutamate plays an "endocrine-like" role as part of a larger homeostatic system.
GABA precursor
Glutamate also serves as the precursor for the synthesis of the inhibitory GABA in GABA-ergic neurons. This reaction is catalyzed by glutamate decarboxylase (GAD), which is most abundant in the cerebellum and pancreas.
Stiff-man syndrome is a neurologic disorder caused by anti-GAD antibodies, leading to a decrease in GABA synthesis and, therefore, impaired motor function such as muscle stiffness and spasm. Since the pancreas is also abundant for the enzyme GAD, a direct immunological destruction occurs in the pancreas and the patients will have diabetes mellitus.
Flavor enhancer
Main article: Glutamic acid (flavor)Free glutamic acid is present in a wide variety of foods, including cheese and soy sauce, and is responsible for umami, one of the five basic tastes of the human sense of taste. Glutamic acid is often used as a food additive and flavour enhancer in the form of its sodium salt monosodium glutamate (MSG).
Nutrient
All meats, poultry, fish, eggs, dairy products, and kombu are excellent sources of glutamic acid. Some protein-rich plant foods also serve as sources. Thirty to 35% of the protein in wheat is glutamic acid. Ninety-five percent of the dietary glutamate is metabolized by intestinal cells in a first pass.[19]
Plant growth
Auxigro is a plant growth preparation that contains 30% glutamic acid.
NMR Spectroscopy
In recent years, there has been much research into the use of RDCs in NMR spectroscopy. A glutamic acid derivative, poly-γ-benzyl-L-glutamate (PBLG), is often used as an alignment medium to control the scale of the dipolar interactions observed.[20]
Production
China-based Fufeng Group Limited is the largest producer of glutamic acid in the world, with capacity increasing to 300,000 tons at the end of 2006 from 180,000 tons during 2006, putting them at 25%–30% of the Chinese market. Meihua is the second-largest Chinese producer. Together, the top-five producers have roughly 50% share in China. Chinese demand is roughly 1.1 million tons per year, while global demand, including China, is 1.7 million tons per year.
Pharmacology
The drug phencyclidine (more commonly known as PCP) antagonizes glutamic acid non-competitively at the NMDA receptor. For the same reasons, sub-anaesthetic doses of ketamine have strong dissociative and hallucinogenic effects. Glutamate does not easily pass the blood brain barrier, but, instead, is transported by a high-affinity transport system.[21] It can also be converted into glutamine.
See also
References
- ^ http://hazard.com/msds/mf/baker/baker/files/g3970.htm
- ^ a b c Robert Sapolsky (2005). "Biology and Human Behavior: The Neurological Origins of Individuality, 2nd edition". The Teaching Company. "see pages 19 and 20 of Guide Book"
- ^ Renton, Alex (2005-07-10). "If MSG is so bad for you, why doesn't everyone in Asia have a headache?". The Guardian. http://observer.guardian.co.uk/foodmonthly/story/0,,1522368,00.html. Retrieved 2008-11-21.
- ^ "Kikunae Ikeda Sodium Glutamate". Japan Patent Office. 2002-10-07. http://www.jpo.go.jp/seido_e/rekishi_e/kikunae_ikeda.htm. Retrieved 2008-11-21.
- ^ a b [Grabowska A, Nowicki M, Kwinta J (2011). "Glutamate dehydrogenase of the germinating triticale seeds: gene expression, activity distribution and kinetic characteristics". Acta Phys. Plant. 2011 (5): 1981. doi:10.1007/s11738-011-0801-1. http://www.springerlink.com/content/m05q717303575376/.]
- ^ McEntee, W. & Crook, T (1993). "Glutamate: its role in learning, memory, and the aging brain". Psychopharmacology 111 (4): 391–401. doi:10.1007/BF02253527. PMID 7870979. http://www.ncbi.nlm.nih.gov/pubmed/7870979.
- ^ Okubo Y, Sekiya H, Namiki S, Sakamoto H, Iinuma S, Yamasaki M, Watanabe M, Hirose K, Iino M. et al. (2010). "Imaging extrasynaptic glutamate dynamics in the brain". Proc Natl Acad Sci U S A 107: 6526–6531. doi:10.1073/pnas.0913154107. PMID 20308566.
- ^ Shigeri Y, Seal RP, Shimamoto K (July 2004). "Molecular pharmacology of glutamate transporters, EAATs and VGLUTs". Brain Res. Brain Res. Rev. 45 (3): 250–65. doi:10.1016/j.brainresrev.2004.04.004. PMID 15210307.
- ^ Manev H, Favaron M, Guidotti A, Costa E (July 1989). "Delayed increase of Ca2+ influx elicited by glutamate: role in neuronal death". Mol. Pharmacol. 36 (1): 106–12. PMID 2568579. http://molpharm.aspetjournals.org/cgi/pmidlookup?view=long&pmid=2568579.
- ^ Hynd MR, Scott HL, Dodd PR. (October 2004). "Glutamate-mediated excitotoxicity and neurodegeneration in Alzheimer's disease". Neurochem Int. 45 (5): 583–95. doi:10.1016/j.neuint.2004.03.007. PMID 15234100. http://www.ncbi.nlm.nih.gov/pubmed/15234100.
- ^ Okumoto, S., et al. (2005). "Detection of glutamate release from neurons by genetically encoded surface-displayed FRET nanosensors". Proceedings of the National Academy of Sciences U.S.A 102 (24): 8740–8745. doi:10.1073/pnas.0503274102. PMC 1143584. PMID 15939876. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1143584.
- ^ Ellis-Davies, G.C.R., et al. (2007). "4- Carboxymethoxy-5,7-dinitroindolinyl-Glu: an improved caged glutamate for expeditious ultra-violet and 2-photon photolysis in brain slices". Journal of Neuroscience 27 (June): 6601–6604. doi:10.1523/JNEUROSCI.1519-07.2007. PMID 17581946.
- ^ Laughton, D.L.; Wheeler, S.V.; Lunt, G.G.; Wolstenholme, A.J. (1995). "The beta-subunit of Caenorhabditis elegans avermectin receptor responds to glycine and is encoded by chromosome 1"". J. Neurochem 64 (5): 2354–2357. doi:10.1046/j.1471-4159.1995.64052354.x. PMID 7536811.
- ^ Cully, D.F.; Vassilatis, D.K.; Liu, K.K.; Paress, P.S.; Van der Ploeg, LHT; Schaeffer, J.M.; Arena, J.P. (1994). "Cloning of an avermectin-sensitive glutamate gated choride channels from Caenorhabditis elegans"". Nature 371 (6499): 707–711. doi:10.1038/371707a0. PMID 7935817.
- ^ Cully, D.F., Paress, P.S., Liu, K.K., Schaeffer, J.M. and Arena, J.P. 1996. "Identification of a Drosophila melanogaster glutamate-gated chloride channel sensitive to the antiparasitic agent avermectin". J. Biol. Chem. '271, 20187-20191'
- ^ Tribble, N.D.; Burka, J.F.; Kibenge, F.S.B. (2007). "Identification of the genes encoding for putative gamma aminobutyric acid (GABA) and glutamate-gated chloride channel (GluCl) alpha receptor subunits in sea lice (Lepeophtheirus salmonis)"". J. Vet. Pharmacol. Ther. 30 (2): 163–167. doi:10.1111/j.1365-2885.2007.00823.x. PMID 17348903.
- ^ a b Augustin H, Grosjean Y, Chen K, Sheng Q, Featherstone DE (2007). "Nonvesicular Release of Glutamate by Glial xCT Transporters Suppresses Glutamate Receptor Clustering In Vivo". Journal of Neuroscience 27 (1): 111–123. doi:10.1523/JNEUROSCI.4770-06.2007. PMC 2193629. PMID 17202478. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2193629.
- ^ Zheng Xi, Baker DA, Shen H, Carson DS, Kalivas PW (2002). "Group II metabotropic glutamate receptors modulate extracellular glutamate in the nucleus accumbens". Journal of Pharmacology and Experimental Therapeutics 300 (1): 162–171. doi:10.1124/jpet.300.1.162. PMID 11752112.
- ^ Reeds, P.J., et al. (1 April 2000). "Intestinal glutamate metabolism". Journal of Nutrition 130 (4s): 978S–982S. PMID 10736365. http://jn.nutrition.org/cgi/content/full/130/4/978S.
- ^ C. M. Thiele, Concepts Magn. Reson. A, 2007, 30A, 65-80
- ^ Smith QR (April 2000). "Transport of glutamate and other amino acids at the blood-brain barrier". J. Nutr. 130 (4S Suppl): 1016S–22S. PMID 10736373. http://jn.nutrition.org/cgi/pmidlookup?view=long&pmid=10736373.
Further reading
- Nelson, David L.; Cox, Michael M. (2005), Principles of Biochemistry (4th ed.), New York: W. H. Freeman, ISBN 0-7167-4339-6
The 20 common amino acids By properties AliphaticBranched-chain amino acids (Valine · Isoleucine · Leucine) · Methionine · Alanine · Proline · GlycineAromaticPolar, unchargedPositive charge (pKa)Negative charge (pKa)GeneralOther classifications biochemical families: prot · nucl · carb (glpr, alco, glys) · lipd (fata/i, phld, strd, gllp, eico) · amac/i · ncbs/i · ttpy/iGlutamatergics Ionotropic Agonists: 5-Fluorowillardiine • AMPA • Domoic acid • Quisqualic acid; Positive allosteric modulators: Aniracetam • Cyclothiazide • CX-516 • CX-546 • CX-614 • CX-691 • CX-717 • Diazoxide • HCTZ • IDRA-21 • LY-392,098 • LY-404,187 • LY-451,395 • LY-451,646 • LY-503,430 • Oxiracetam • PEPA • Piracetam • Pramiracetam • S-18986 • Sunifiram • Unifiram
Antagonists: ATPO • Barbiturates • Caroverine • CNQX • DNQX • GYKI-52466 • NBQX • Perampanel • Talampanel • Tezampanel • Topiramate; Negative allosteric modulators: GYKI-53,655Agonists: Glutamate/acite site competitive agonists: Aspartate • Glutamate • Homoquinolinic acid • Ibotenic acid • NMDA • Quinolinic acid • Tetrazolylglycine; Glycine site agonists: ACBD • ACPC • ACPD • Alanine • CCG • Cycloserine • DHPG • Fluoroalanine • Glycine • HA-966 • L-687,414 • Milacemide • Sarcosine • Serine • Tetrazolylglycine; Polyamine site agonists: Acamprosate • Spermidine • Spermine
Antagonists: Competitive antagonists: AP5 (APV) • AP7 • CGP-37849 • CGP-39551 • CGP-39653 • CGP-40116 • CGS-19755 • CPP • LY-233,053 • LY-235,959 • LY-274,614 • MDL-100,453 • Midafotel (d-CPPene) • NPC-12,626 • NPC-17,742 • PBPD • PEAQX • Perzinfotel • PPDA • SDZ-220581 • Selfotel; Noncompetitive antagonists: ARR-15,896 • Caroverine • Dexanabinol • FPL-12495 • FR-115,427 • Hodgkinsine • Magnesium • MDL-27,266 • NPS-1506 • Psychotridine • Zinc; Uncompetitive pore blockers: 2-MDP • 3-MeO-PCP • 8A-PDHQ • Alaproclate • Amantadine • Aptiganel • ARL-12,495 • ARL-15,896-AR • ARL-16,247 • Budipine • Delucemine • Dexoxadrol • Dextrallorphan • Dieticyclidine • Dizocilpine • Endopsychosin • Esketamine • Etoxadrol • Eticyclidine • Gacyclidine • Ibogaine • Indantadol • Ketamine • Ketobemidone • Loperamide • Memantine • Meperidine (Pethidine) • Methadone • Methorphan (Dextromethorphan, Levomethorphan) • Methoxetamine • Milnacipran • Morphanol (Dextrorphan, Levorphanol) • NEFA • Neramexane • Nitrous oxide • Noribogaine • Orphenadrine • PCPr • Phencyclamine • Phencyclidine • Propoxyphene • Remacemide • Rhynchophylline • Riluzole • Rimantadine • Rolicyclidine • Sabeluzole • Tenocyclidine • Tiletamine • Tramadol • Xenon; Glycine site antagonists: ACEA-1021 • ACEA-1328 • ACPC • Carisoprodol • CGP-39653 • CKA • DCKA • Felbamate • Gavestinel • GV-196,771 • Kynurenic acid • L-689,560 • L-701,324 • Lacosamide • Licostinel • LU-73,068 • MDL-105,519 • Meprobamate • MRZ 2/576 • PNQX • ZD-9379; NR2B subunit antagonists: Besonprodil • CO-101,244 (PD-174,494) • CP-101,606 • Eliprodil • Haloperidol • Ifenprodil • Isoxsuprine • Nylidrin • Ro8-4304 • Ro25-6981 • Traxoprodil; Polyamine site antagonists: Arcaine • Co 101676 • Diaminopropane • Acamprosate • Diethylenetriamine • Huperzine A • Putrescine • Ro 25-6981; Unclassified/unsorted antagonists: Chloroform • Diethyl ether • Enflurane • Ethanol (Alcohol) • Halothane • Isoflurane • Methoxyflurane • Toluene • Trichloroethane • Trichloroethanol • Trichloroethylene • XyleneAgonists: 5-Iodowillardiine • ATPA • Domoic acid • Kainic acid • LY-339,434 • SYM-2081
Antagonists: CNQX • DNQX • LY-382,884 • NBQX • NS102 • Tezampanel • Topiramate • UBP-302; Negative allosteric modulators: NS-3763Metabotropic Agonists: Unselective: ACPD • DHPG • Quisqualic acid; mGlu1-selective: Ro01-6128 • Ro67-4853 • Ro67-7476 • VU-71; mGlu5-selective: ADX-47273 • CDPPB • CHPG • DFB • VU-1545
Antagonists: Unselective: MCPG • NPS-2390; mGlu1-selective: BAY 36-7620 • CPCCOEt • LY-367,385 • LY-456,236; mGlu5-selective: Dipraglurant • DMeOB • Fenobam • LY-344,545 • MPEP • MTEP • SIB-1757 • SIB-1893Agonists: Unselective: L-AP4; mGlu4-selective: PHCCC • VU-001,171 • VU-0155,041; mGlu7-selective: AMN082; mGlu8-selective: DCPG
Antagonists: Unselective: CPPG • MAP4 • MSOP • MPPG • MTPG • UBP-1112; mGlu7-selective: MMPIPTransporter
inhibitorsDHKA • PDC • WAY-213,613vGluTs7-CKA • Evans blueK→acetyl-CoA G G→pyruvate→citrateG→glutamate→
α-ketoglutarateotherα-Ketoisovaleric acid · Isobutyryl-CoA · Methacrylyl-CoA · 3-Hydroxyisobutyryl-CoA · 3-Hydroxyisobutyric acid · 2-Methyl-3-oxopropanoic acidG→fumarateG→oxaloacetatesee urea cycleOther biochemical families: prot · nucl · carb (glpr, alco, glys) · lipd (fata/i, phld, strd, gllp, eico) · amac/i · ncbs/i · ttpy/iNeurotransmitters Amino acids Alanine · Aspartate · Cycloserine · DMG · GABA · Glutamate · Glycine · Hypotaurine · Kynurenic acid (Transtorine) · NAAG (Spaglumic acid) · NMG (Sarcosine) · Serine · Taurine · TMG (Betaine)
Endocannabinoids 2-AG · 2-AGE (Noladin ether) · AEA (Anandamide) · NADA · OAE (Virodhamine) · Oleamide · PEA (Palmitoylethanolamide) · RVD-Hpα · Hp (Hemopressin)
Gasotransmitters Monoamines Purines Trace amines 3-ITA · 5-MeO-DMT · Bufotenin · DMT · NMT · Octopamine · Phenethylamine · Synephrine · Thyronamine · Tryptamine · Tyramine
Others 1,4-BD · Acetylcholine · GBL · GHB · Histamine
See also Template:NeuropeptidesNeurotoxins Animal Poisons & Venoms: Batrachotoxin • Bestoxin • Birtoxin • Bungarotoxin • Charybdotoxin • Conotoxin • Saxitoxin • Tetrodotoxin
Neurotoxic drugs: Amphetamine • Lisdexamfetamine • Methamphetamine • αET • αMT • MBDB • MDA • MDEA • MDMA (Ecstasy) • PBA • PCA • PIA • 1,4-BD • GBL • GHB • Ibotenic Acid • Dizocilpine (MK-801) • Ketamine • Phencyclidine (PCP) • 5,7-DHT • 6-OHDA • MPTP/MPP+ • Norsalsolinol • Ethanol (Alcohol)
Bacterial toxins: Botulinum toxin • Tetanospasmin
Fungal toxins: Bicuculline
Plant toxins: Penitrem A • Picrotoxin
Pesticides: Rotenone
Nerve agents: Cyclosarin EA-3148 • GV (nerve agent) • Novichok agent • Sarin • Soman • Tabun (nerve agent) • VE (nerve agent) • VG (nerve agent) • VM (nerve agent) • VR (nerve agent) • VX (nerve agent)
Neurotransmitters and precursors: Dopamine • Glutamate • L-Tyrosine • L-Phenylalanine • L-DOPA (Levodopa) • L-GlutamineCategories:- Amino acids
- Proteinogenic amino acids
- Glucogenic amino acids
- Acidic amino acids
- Dicarboxylic acids
- Neurotransmitters
- Flavour enhancers
- Umami enhancers
- Glutamates
Wikimedia Foundation. 2010.

