- Acamprosate
-
Acamprosate 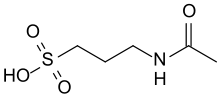
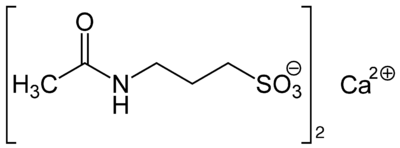
Systematic (IUPAC) name 3-Acetamidopropane-1-sulfonic acid Clinical data Pregnancy cat. C[1] (US) B2 (AU) Legal status POM (UK) ℞-only (US) Routes Oral (333mg tablets of acamprosate calcium)[1] Pharmacokinetic data Bioavailability 11%[1] Protein binding Negligible[1] Metabolism Nil[1] Half-life 20 to 33 hours[1] Excretion Renal[1] Identifiers CAS number 77337-76-9 
ATC code N07BB03 PubChem CID 155434 DrugBank APRD00661 ChemSpider 136929 
UNII N4K14YGM3J 
KEGG D07058 
ChEBI CHEBI:51042 
ChEMBL CHEMBL1201293 
Chemical data Formula C5H11NO4S Mol. mass 181.211 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Acamprosate, also known as N-acetyl homotaurine[2] and by the brand name Campral, is a drug used for treating alcohol dependence.
Acamprosate is thought to stabilize the chemical balance in the brain that would otherwise be disrupted by alcoholism, possibly by blocking glutamatergic N-methyl-D-aspartate receptors, while gamma-aminobutyric acid (GABA) type A receptors are activated.[3] Reports indicate that acamprosate only works with a combination of attending support groups and abstinence from alcohol.[4][5] Certain serious side effects include diarrhea, allergic reactions, irregular heartbeats, and low or high blood pressure, while less serious side effects include headaches, insomnia, and impotence.[6] Acamprosate should not be taken by people with kidney problems or allergies to the drug.[7]
Campral is manufactured and marketed in the United States by Forest Laboratories, while Merck KGaA markets it outside the US. It is sold as 333 mg white and odorless tablets of acamprosate calcium, which is the equivalent of 300 mg of acamprosate.[1]
Contents
Physiological action
Alcohol inhibits activity of biochemical receptors called N-methyl-D-aspartate receptors, or NMDARs, so that chronic alcohol consumption leads to the overproduction (upregulation) of these receptors . Thereafter, sudden alcohol abstinence causes the excessive numbers of NMDARs to be more active than normal and to produce the symptoms of delirium tremens and excitotoxic neuronal death.[8] Withdrawal from alcohol induces a surge in release of excitatory neurotransmitters like glutamate, which activates NMDARs.[9] Acamprosate reduces this glutamate surge.[10] The drug also protects cultured cells from excitotoxicity induced by ethanol withdrawal[11] and from glutamate exposure combined with ethanol withdrawal.[12]
Possible neuroprotection
In addition to its apparent ability to help patients refrain from drinking, some evidence suggests that acamprosate is neuroprotective (that is, it protects neurons from damage and death caused by the effects of alcohol withdrawal, or possibly other insults).[2][10] For example, acamprosate has been found to protect cultured cells from damage induced by ischemia (inadequate blood flow).[13] The drug also protected infant hamsters from brain damage induced by injections of the toxin ibotenic acid (which exacerbates excitotoxicity, the harmful over-activation of glutamate receptors).[14]
One Brazilian study has shown that Acamprosate may be an effective treatment for tinnitus (persistent ringing in the ears due to hearing loss).[15]
Approval
While the Food and Drug Administration (FDA) in the United States approved this drug in July 2004, it has been legal in Europe since 1989. After it approved the drug, the FDA released this statement:
While its mechanism of action is not fully understood, Campral is thought to act on the brain pathways related to alcohol abuse. Campral was demonstrated to be safe and effective by multiple placebo-controlled clinical studies involving alcohol-dependent patients who had already been withdrawn from alcohol, (i.e., detoxified). Campral proved superior to placebo in maintaining abstinence (keeping patients off alcohol consumption), as indicated by a greater percentage of acamprosate-treated subjects being assessed as continuously abstinent throughout treatment. Campral is not addicting and was generally well-tolerated in clinical trials. The most common adverse events reported for patients taking Campral included headache, diarrhea, flatulence, and nausea.[16]
Clinical study results
The Scripps Research Institute conducted a double blind study comparing acamprosate and placebos, in combination with psychotherapy, in the effectiveness of treating alcohol dependence. The researchers concluded that acamprosate is "safe and effective" as acamprosate increased the percentage of alcohol-free days.[17]
Another study was conducted by Princess Alexandra Hospital in Brisbane comparing the use of acamprosate, naltrexone, or both drugs at once (with each pharmacological treatment also paired with cognitive behavioral therapy) in a twelve-week study.[18] This study concluded that a combination of medications was generally more popular and yielded better results than using either drug alone, as outlined below.
Percentage attending program Abstinence rates Average number of days abstinence1 Days until first breach of abstinence1 Acamprosate group 66.1% 50.8% 45.07 days 26.79 days Naltrexone group 79.7% 66.1% 49.95 days 26.7 days Drug combination group 83.1% 67.8% 53.58 days 37.32 days - 1 This statistic applies to patients who could not remain abstinent throughout the entire 84-day period.
References
- ^ a b c d e f g h "Campral Description" (PDF). Archived from the original on 2006-03-18. http://web.archive.org/web/20060318125724/http://www.fda.gov/cder/foi/label/2004/21431lbl.pdf. Retrieved 2006-04-02.
- ^ a b Mann K, Kiefer F, Spanagel R, Littleton J (July 2008). "Acamprosate: recent findings and future research directions". Alcohol. Clin. Exp. Res. 32 (7): 1105–10. doi:10.1111/j.1530-0277.2008.00690.x. PMID 18540918.
- ^ Williams, SH. (2005). "Medications for treating alcohol dependence". American Family Physician 72 (9): 1775–1780. PMID 16300039. http://www.aafp.org/afp/20051101/1775.html.
- ^ Mason, BJ (2001). "Treatment of alcohol-dependent outpatients with acamprosate: a clinical review.". The Journal of clinical psychiatry 62 Suppl 20: 42–8. PMID 11584875.
- ^ GABA Agonist (Acamprosate) for Alcohol Treatment, Alcohol Rehab Thailand
- ^ "Acamprosate". drugs.com. 2005-03-25. http://www.drugs.com/mtm/acamprosate.html. Retrieved 2007-01-08.
- ^ "Acamprosate Oral - Who should not take this medication?". WebMD.com. http://www.webmd.com/drugs/drug-91488-Acamprosate+Oral.aspx?drugid=91488&drugname=Acamprosate+Oral&pagenumber=5. Retrieved 2007-01-08.
- ^ Tsai, G; Coyle, JT (1998). "The role of glutamatergic neurotransmission in the pathophysiology of alcoholism". Annual review of medicine 49: 173–84. doi:10.1146/annurev.med.49.1.173. PMID 9509257.
- ^ Tsai, GE; Ragan, P; Chang, R; Chen, S; Linnoila, VM; Coyle, JT (1998). "Increased glutamatergic neurotransmission and oxidative stress after alcohol withdrawal". The American journal of psychiatry 155 (6): 726–32. PMID 9619143. http://ajp.psychiatryonline.org/cgi/content/full/155/6/726.
- ^ a b De Witte, P; Littleton, J; Parot, P; Koob, G (2005). "Neuroprotective and abstinence-promoting effects of acamprosate: elucidating the mechanism of action". CNS drugs 19 (6): 517–37. PMID 15963001.
- ^ Mayer, S; Harris, BR; Gibson, DA; Blanchard, JA; Prendergast, MA; Holley, RC; Littleton, J (2002). "Acamprosate, MK-801, and ifenprodil inhibit neurotoxicity and calcium entry induced by ethanol withdrawal in organotypic slice cultures from neonatal rat hippocampus". Alcoholism, clinical and experimental research 26 (10): 1468–78. doi:10.1097/01.ALC.0000033261.14548.D2. PMID 12394279.
- ^ Al Qatari, M; Khan, S; Harris, B; Littleton, J (2001). "Acamprosate is neuroprotective against glutamate-induced excitotoxicity when enhanced by ethanol withdrawal in neocortical cultures of fetal rat brain". Alcoholism, clinical and experimental research 25 (9): 1276–83. doi:10.1111/j.1530-0277.2001.tb02348.x. PMID 11584146.
- ^ Engelhard, K; Werner C, Lu H, Mollenberg O, Zieglgansberger W, Kochs E (2006). "The neuroprotective effect of the glutamate antagonist acamprosate following experimental cerebral ischemia. A study with the lipid peroxidase inhibitor u-101033e". Anaesthesist 49 (9): 816–821. PMID 11076270.
- ^ Adde-Michel, C; Hennebert O, Laudenbach V, Marret S, Leroux P (2005). "Effect of acamprosate on neonatal excitotoxic cortical lesions in in utero alcohol-exposed hamsters". Neuroscience Letters 374 (2): 109–112. doi:10.1016/j.neulet.2004.10.037. PMID 15644274.
- ^ Azevedo AA, Figueiredo RR (2005). "Tinnitus treatment with acamprosate: double-blind study". Braz J Otorhinolaryngol 71 (5): 618–23. doi:/S0034-72992005000500012. PMID 16612523.
- ^ "FDA Approves New Drug for Treatment of Alcoholism". FDA Talk Paper. Food and Drug Administration. 2004-07-29. Archived from the original on 2008-01-17. http://web.archive.org/web/20080117175319/http://www.fda.gov/bbs/topics/answers/2004/ANS01302.html. Retrieved 2009-08-15.
- ^ Mason, BJ; Goodman AM, Chabac S, Lehert P (2006). "Effect of oral acamprosate on abstinence in patients with alcohol dependence in a double-blind, placebo-controlled trial: The role of patient motivation". J Psychiatr Res 40 (5): 383–393. doi:10.1016/j.jpsychires.2006.02.002. PMID 16546214.
- ^ Feeney, GF; Connor JP, Young RM, Tucker J, McPherson A (2006). "Combined acamprosate and naltrexone, with cognitive behavioural therapy is superior to either medication alone for alcohol abstinence: A single centre's experience with pharmacotherapy". Alcohol Alcohol 41 (3): 321–327. doi:10.1093/alcalc/agl007. PMID 16467406.
Antiaddictives (N07B) Nicotine dependence/
(Nicotinic agonist)Nicotine • Dianicline • Varenicline • Lobeline • Mecamylamine • Scopolamine
NDRI (Bupropion) • AA (Clonidine) • CB1 (Surinabant)Alcohol dependence AD inhibitor (Disulfiram, Calcium carbimide) • mGluR (Acamprosate) • Opioid receptor antagonists (Naltrexone, Nalmefene) • Topiramate • AA (Clonidine) • BaclofenOpioid dependence Stimulant dependence Benzodiazepine dependence Cocaine dependence Sedative-Hypnotic dependence Categories:- Alcohol abuse
- Drugs
- Sulfonic acids
- Acetamides
Wikimedia Foundation. 2010.