- Phenytoin
-
Phenytoin 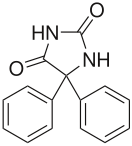
Systematic (IUPAC) name 5,5-diphenylimidazolidine-2,4-dione Clinical data Trade names Dilantin AHFS/Drugs.com monograph MedlinePlus a682022 Pregnancy cat. D Legal status ℞ Prescription only Routes Oral, parenteral Pharmacokinetic data Bioavailability 70-100% oral, 24.4% for rectal and intravenous administration Protein binding 90% Metabolism hepatic Half-life 6–24 hours Excretion Primarily through the bile, urinary Identifiers CAS number 57-41-0 
ATC code N03AB02 N03AB04, N03AB05 PubChem CID 1775 DrugBank DB00252 ChemSpider 1710 
UNII 6158TKW0C5 
KEGG D00512 
ChEBI CHEBI:8107 
ChEMBL CHEMBL16 
Chemical data Formula C15H12N2O2 Mol. mass 252.268 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Phenytoin sodium is a commonly used antiepileptic. Phenytoin (fœnit'oin, IPA) acts to suppress the abnormal brain activity seen in seizure by reducing electrical conductance among brain cells by stabilizing the inactive state of voltage-gated sodium channels. Aside from seizures, it is an option in the treatment of trigeminal neuralgia in the event that carbamazepine or other first-line treatment seems inappropriate.
It is sometimes considered a class 1b antiarrhythmic.[1]
Contents
Trade names
Phenytoin sodium has been marketed as Phenytek by Mylan Laboratories, previously Bertek Pharmaceuticals, and Dilantin; Australia also Dilantin Kapseals and Dilantin Infatabs in the USA, Eptoin by Abbott Group in India and as Epanutin in the UK and Israel, by Parke-Davis, now part of Pfizer. In the USSR and post-USSR countries, it was/is marketed as Дифенин (Diphenin, Dipheninum),.
History
Phenytoin (diphenylhydantoin) was first synthesized by German chemist Heinrich Biltz in 1908.[2] Biltz sold his discovery to Parke-Davis, which did not find an immediate use for it. In 1938, outside scientists including H. Houston Merritt and Tracy Putnam discovered phenytoin's usefulness for controlling seizures, without the sedative effects associated with phenobarbital.
According to Goodman and Gilman's Pharmacological Basis of Therapeutics,
- In contrast to the earlier accidental discovery of the antiseizure properties of bromide and phenobarbital, phenytoin was the product of a search among nonsedative structural relatives of phenobarbital for agents capable of suppressing electroshock convulsions in laboratory animals.[3]
There are some indications that phenytoin has other effects, including anxiety control and mood stabilization, although it has never been approved for those purposes by the FDA. Jack Dreyfus, founder of the Dreyfus Fund, became a major proponent of phenytoin as a means to control nervousness and depression when he received a prescription for Dilantin in 1966. He is believed to have supplied large amounts of the drug to Richard Nixon throughout the late 1960s and early 1970s. Dreyfus' experience with phenytoin is outlined in his book, A Remarkable Medicine Has Been Overlooked,.[4] Despite more than $70 million in personal financing, his push to see phenytoin evaluated for alternative uses has had little lasting effect on the medical community. This was partially because Parke-Davis was reluctant to invest in a drug nearing the end of its patent life, and partially due to mixed results from various studies. It was approved by the USA Food and Drug Administration in 1953 for use in seizures.
Dilantin made an appearance in the 1962 novel One Flew Over the Cuckoo's Nest by Ken Kesey, both as an anticonvulsant and as a mechanism to control inmate behavior.
In 2008, the drug was put on the FDA's Potential Signals of Serious Risks List to be further evaluated for approval. The list means that the FDA has identified a potential safety issue, but does not mean that FDA has identified a causal relationship between the drug and the listed risk.
According to the FDA's New Safety Information Identified by the Adverse Event Reporting System (AERS) Phenytoin Injection (Dilantin) has been associated with the risk of Purple Glove Syndrome.
Side-effects
Neurologic
At therapeutic doses, phenytoin produces horizontal gaze nystagmus. At toxic doses, patients experience sedation, cerebellar ataxia, and ophthalmoparesis, as well as seizures. Idiosyncratic side-effects of phenytoin, as with other anticonvulsants, include rash and severe allergic reactions.
Phenytoin may accumulate in the cerebral cortex over long periods of time, as well as causing atrophy of the cerebellum when administered at chronically high levels. Despite this, the drug has a long history of safe use, making it one of the more popular anti-convulsants prescribed by doctors, and a common "first line of defense" in seizure cases.
Hematologic
It has been suggested that phenytoin causes a reduction in folic acid levels, predisposing patients to megaloblastic anemia. Folic acid is presented in foods as polyglutamate, which is then converted into monoglutamates by intestinal conjugase. Phenytoin acts by inhibiting this enzyme, thereby causing folate deficiency.[5] Other side effects may include: agranulocytosis, aplastic anemia, leukopenia[citation needed], thrombocytopenia.
Teratogenicity
Phenytoin is a known teratogen. The syndrome consists of craniofacial anomalies (broad nasal bridge, cleft lip and palate, microcephaly) and a mild form of mental retardation (average IQ=71).[6] This syndrome resembles the well-described Fetal Alcohol Syndrome[7] and has also been called the "fetal hydantoin syndrome".[citation needed] Data now being collected by the Epilepsy and Antiepileptic Drug Pregnancy Registry may one day answer this question definitively.
Some recommend avoiding polytherapy and maintaining the minimal dose possible during pregnancy, but acknowledge that current data do not provide clear answers.[8]
Carcinogenicity
There is no good evidence that phenytoin is a human carcinogen.[9][10]
Gingival
Phenytoin has been associated with drug-induced gingival enlargement (overgrowth of the gums), probably due to above-mentioned folate deficiency; indeed, evidence from a randomized controlled trial suggests that folic acid supplementation can prevent gingival enlargement in children who take phenytoin.[11] Plasma concentrations needed to induce gingival lesions has not been clearly defined. Effects consist of the following: bleeding upon probing, increased gingival exudate, pronounced gingival inflammatory response to plaque levels, associated in some instances with bone loss but without tooth detachment.
Suicide risk
Following almost 200 studies of 11 anti-seizure drugs, the FDA has also warned of an increased suicide risk for any patients treated with certain anti-seizure drugs. The study of 44,000 patients found that patients whose epilepsy is treated with drugs face about twice the risk of suicidal thoughts compared to placebo-takers. Although phenytoin was not named in the study, the FDA announced that it expected the risk applied to every epilepsy drug.[12]
Dermatologic
Hypertrichosis, rash, exfoliative dermatitis, pruritis.
In autoimmune disease
Phenytoin has been known to cause drug-induced lupus.[13]
Phenytoin therapy has been linked to the life-threatening skin reactions Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN). These conditions are significantly more common in patients with a particular HLA-B allele, HLA-B*1502.[14] This allele occurs almost exclusively in patients with ancestry across broad areas of Asia, including South Asian Indians.
In immunodeficiency disease
Phenytoin is also associated with induction of reversible IgA deficiency.[14]
Interactions
Phenytoin is an inducer of the CYP3A4 and CYP2C19 families of the P450 enzyme responsible for the hepatic degradation of various drugs.
Warfarin (Coumadin) increases serum phenytoin levels and prolongs the serum half-life of phenytoin by inhibiting its metabolism.
Pharmacokinetic
Phenytoin kinetics are nonlinear and saturable, resulting in highly variable concentrations even with minor dosage changes. A small increase in dose may lead to a large increase in drug concentration as elimination becomes saturated.
Chemistry
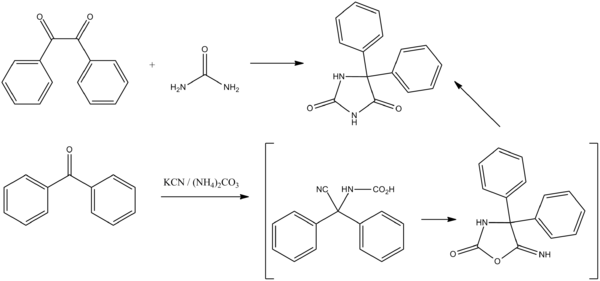
Phenytoin, 5,5-diphenylimidazolidinedione is synthesized in two different ways. The first involves a base catalyzed addition of Urea to benzil followed by a benzilic acid rearrangement (1,2 phenyl migration) to form the desired product. This is known as the Biltz Synthesis of phenytoin.[2]
The second method involves the reaction of benzophenone with sodium cyanide in the presence of ammonium carbonate, followed by the simultaneous cyclization of the resulting product (carboxyaminonitrile) and its rearrangement under the reaction conditions to form phenytoin.
- US patent 2409754, Hense, H. R., "Method for obtaining hydantoins", issued 1946-10-22, assigned to Parke Davis
References
- ^ Balaji S (October 2004). "Medical therapy for sudden death". Pediatr. Clin. North Am. 51 (5): 1379–87. doi:10.1016/j.pcl.2004.04.002. PMID 15331289. http://linkinghub.elsevier.com/retrieve/pii/S0031395504000513.
- ^ a b Biltz, H. (1908). "Constitution of the Products of the Interaction of Substituted Carbamides on Benzil and Certain New Methods for the Preparation of 5,5-Diphenylhydantoin". Chemische Berichte 41: 1379–93.
- ^ Goodman and Gilman's Pharmacological Basis of Therapeutics, 10th ed. (New York: McGraw-Hill, 2001).
- ^ Dreyfus, Jack (1998). A Remarkable Medicine Has Been Overlooked: Including an Autobiography and the Clinical Section of the Broad Range of Use of Phenytoin. Continuum International Publishing Group. ISBN 0-8264-1069-3.
- ^ Carl GF, Smith ML (1992). "Phenytoin-folate interactions: differing effects of the sodium salt and the free acid of phenytoin". Epilepsia 33 (2): 372–5. doi:10.1111/j.1528-1157.1992.tb02330.x. PMID 1547769.
- ^ Beckmann, Charles R., et al. (2002). Obstetrics and Gynecology (4th ed.). Baltimore: Lippincott Williams & Wilkins.
- ^ CDC. (2004). Fetal Alcohol Syndrome: Guidelines for Referral and Diagnosis. Can be downloaded at http://www.cdc.gov/fas/faspub.htm.
- ^ Adab N, Tudur SC, Vinten J, Williamson P, Winterbottom J (2004). Adab, Naghme. ed. "Common antiepileptic drugs in pregnancy in women with epilepsy". Cochrane Database Syst Rev (3): CD004848. doi:10.1002/14651858.CD004848. PMID 15266543.
- ^ Report on Carcinogens, Eleventh Edition (PB2005-104914, 2004) p III-216.
- ^ Maeda T, Sano N, Togei K, Shibata M, Izumi K, Otsuka H (1988). "Lack of carcinogenicity of phenytoin in (C57BL/6 x C3H)F1 mice". J Toxicol Environ Health 24 (1): 111–9. doi:10.1080/15287398809531144. PMID 3373561.
- ^ Arya R, Gulati S, Kabra M, Sahu JK, Kalra V (Apr 2011). "Folic acid supplementation prevents phenytoin-induced gingival overgrowth in children". Neurology 76 (15): 1338–43. doi:10.1212/WNL.0b013e3182152844. PMC 3090066. PMID 21482950. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3090066.
- ^ FDA warns of risks from epilepsy drugs.. http://news.yahoo.com/s/ap/20080201/ap_on_he_me/epilepsy_drugs
- ^ Scheinfeld N (August 2003). "Phenytoin in cutaneous medicine: its uses, mechanisms and side effects". Dermatol. Online J. 9 (3): 6. PMID 12952753. http://dermatology.cdlib.org/93/reviews/dilantin/scheinfeld.html.
- ^ a b Man CB, Kwan P, Baum L, et al. (May 2007). "Association between HLA-B*1502 allele and antiepileptic drug-induced cutaneous reactions in Han Chinese". Epilepsia 48 (5): 1015–8. doi:10.1111/j.1528-1167.2007.01022.x. PMID 17509004.
External links
- Medicines for Epilepsy: Dilantin Epilepsy Foundation (broken link).
- Remarkable Medicine, a website about the Dreyfus Foundation's work to expand the indications for phenytoin
- Phenytoin Pharmacokinetics (not a public link)
Anticonvulsants (N03) GABAA receptor agonist Clobazam • Clonazepam • Clorazepate • Diazepam# • Flutoprazepam • Lorazepam • Midazolam • Nimetazepam • Nitrazepam • TemazepamOther GABA agents Carbonic anhydrase inhibitor Channel blockers Primarily sodiumPrimarily calciumUnknown/ungroupedChannel openers PotassiumRetigabineIndirect GABA agents GABA transaminase inhibitor: Valproic acid# (Sodium valproate & Valproate semisodium) • Valpromide • Valnoctamide • Valproate pivoxil
GABA reuptake inhibitor: TiagabineUnknown/multiple/
unsortedPropionatesAntiarrhythmic agents (C01B) Channel blockers Amiodarone • Dronedarone • Bretylium • Bunaftine • Dofetilide • Ibutilide • Nifekalant • Sotalol • Tedisamil • Vernakalant • E-4031Receptor agonists
and antagonistsIon transporters Categories:- Antiarrhythmic agents
- Anticonvulsants
- Hydantoins
- Teratogens
- World Health Organization essential medicines
- IARC Group 2B carcinogens
Wikimedia Foundation. 2010.