- Pentobarbital
-
Pentobarbital 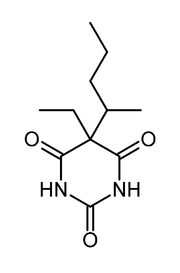
Systematic (IUPAC) name 5-Ethyl-5-(1-methylbutyl)-2,4,6(1H,3H,5H)-pyrimidinetrione Clinical data AHFS/Drugs.com monograph MedlinePlus a682416 Pregnancy cat. D (USA) Legal status USA: Schedule II (oral and parenteral); Schedule III (rectal), UK: Class B Controlled Substance Routes Oral, Intravenous, Intramuscular, Rectal; also Intraperitoneal & Intracardiac (for animal euthanasia) Pharmacokinetic data Bioavailability 70-90% oral; 90% rectal Protein binding 20-45% Metabolism Hepatic Half-life 15-48 hours Excretion Renal Identifiers CAS number 76-74-4 
ATC code N05CA01 QN51AA01 PubChem CID 4737 DrugBank APRD01174 ChemSpider 4575 
UNII I4744080IR 
KEGG D00499 
ChEBI CHEBI:7983 
ChEMBL CHEMBL448 
Chemical data Formula C11H18N2O3 Mol. mass 226.27 SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Pentobarbital is a short-acting barbiturate that was first synthesized in 1928. Pentobarbital is available as both a free acid and a sodium salt, the former of which is only slightly soluble in water and ethanol.[1] One brand name for this drug is Nembutal, coined by Dr. John S. Lundy, who started using it in 1930, from the structural formula of the sodium salt—Na (sodium) + ethyl + methyl + butyl + al (common suffix for barbiturates).[2]
Contents
Uses
Approved
Pentobarbital's FDA-approved human uses include treatment of seizures and preoperative (and other) sedation; it is also approved as a short-term hypnotic.[3]
Unapproved / investigational / off-label
Off-label uses of pentobarbital include reduction of intracranial pressure in Reye's syndrome, traumatic brain injury and induction of coma in cerebral ischemia patients.[3] Pentobarbital-induced coma has been advocated in patients with acute liver failure refractory to mannitol.[4]
Veterinary medicine
In veterinary medicine, sodium pentobarbital is used as an anaesthetic. It is also used by itself, or in combination with complementary agents such as phenytoin, in commercial animal euthanasia injectable solutions. [5]
Human euthanasia
Pentobarbital has also been used for physician-assisted suicide.[citation needed] In the US state of Oregon "oral doses of a barbiturate" have been used for this purpose.[6]
Also in Switzerland[citation needed] and the Netherlands.[citation needed] It was also used in the Northern Territory of Australia, prior to euthanasia becoming illegal in that region.[citation needed]
Capital punishment
Pentobarbital has been approved or considered for use in executions in various U.S. states.[7]
The Danish manufacturer of pentobarbital, Lundbeck, expressed displeasure at this use of their product, and on July 1, 2011, announced they would block sales of the drug to U.S. prisons that carry out the death penalty.
Lundbeck is dedicated to saving people’s lives. Use of our products to end lives contradicts everything we are in business to do. Lundbeck is opposed to the use of its product for the purpose of capital punishment.[8]
They explained this decision with their commitment to UN human rights principles. [7]
Metabolism

Pentobarbital undergoes first-pass metabolism in the liver and possibly the intestines.[9]
Drug interactions
Administration of alcohol, opioids, antihistamines, other sedative-hypnotics, and other central nervous system depressants will cause possible additive effects.[3]
Recreational use
Pentobarbital is a drug that has been used recreationally.[10]
Chemistry
Pentobarbital is synthesized by methods analogous to that of amobarbital, the only difference being that the alkylation of α-ethylmalonic ester is carried out with 2-bromopentane (not 1-bromo-3-methylbutane) to give pentobarbital.[11][12][13]
References
- ^ "Pentobarbital Compound summary (CID4737)". Pubchem. NCBI. http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=4737.
- ^ Fosburgh, L. C. (1997). "From this point in time: Some memories of my part in the history of anesthesia--John S. Lundy, MD". AANA journal 65 (4): 323–328. PMID 9281913.
- ^ a b c "Pentobarbital". Monograph. AHFS / Drugs.com. http://www.drugs.com/monograph/pentobarbital.html.
- ^ Stravitz, R. T.; Kramer, A. H.; Davern, T.; Shaikh, A. O. S.; Caldwell, S. H.; Mehta, R. L.; Blei, A. T.; Fontana, R. J. et al. (2007). "Intensive care of patients with acute liver failure: Recommendations of the U.S. Acute Liver Failure Study Group". Critical Care Medicine 35 (11): 2498–2508. doi:10.1097/01.CCM.0000287592.94554.5F. PMID 17901832.
- ^ "International". Drugs.com. http://www.drugs.com/international/pentobarbital.html.
- ^ "presciption". Death with dignity act - FAQ. Public health Oregon. http://public.health.oregon.gov/ProviderPartnerResources/EvaluationResearch/DeathwithDignityAct/Pages/faqs.aspx#prescription.
- ^ a b "Detailed position (regarding the misuse of pentobarbital)". Lundbeck. http://www.lundbeck.com/global/media/detailed-position.
- ^ "Lundbeck's position regarding the misuse of pentobarbital" (Press release). Lundbeck. July 1, 2011. http://www.lundbeck.com/global/media/lundbecks-position-regarding-the-misuse-of-pentobarbital.
- ^ Knodell, R. G.; Spector, M. H.; Brooks, D. A.; Keller, F. X.; Kyner, W. T. (1980). "Alterations in pentobarbital pharmacokinetics in response to parenteral and enteral alimentation in the rat". Gastroenterology 79 (6): 1211–1216. PMID 6777235.
- ^ Griffiths, R. R.; Johnson, M. W. (2005). "Relative abuse liability of hypnotic drugs: A conceptual framework and algorithm for differentiating among compounds" (pdf). The Journal of clinical psychiatry 66 Suppl 9: 31–41. PMID 16336040. http://neuroscience.jhu.edu/griffiths%20papers/v66s0906.pdf.
- ^ Volwiler, E. H.; Tabern, D. L. (1930). "5,5-SUBSTITUTED BARBITURIC ACIDS". Journal of the American Chemical Society 52 (4): 1676–1679. doi:10.1021/ja01367a061.
- ^ German imperial patent, D.R.P. 293163 (1916), Bayer
- ^ GB patent 650354, Wilde, B. E. & Balaban, I. E., "Improvements in the manufacture of substituted barbituric and thiobarbituric acids", issued 1951-02-21, assigned to Geigy
External links
Anticonvulsants (N03) GABAA receptor agonist Clobazam • Clonazepam • Clorazepate • Diazepam# • Flutoprazepam • Lorazepam • Midazolam • Nimetazepam • Nitrazepam • TemazepamOther GABA agents Carbonic anhydrase inhibitor Channel blockers Primarily sodiumPrimarily calciumUnknown/ungroupedChannel openers PotassiumRetigabineIndirect GABA agents GABA transaminase inhibitor: Valproic acid# (Sodium valproate & Valproate semisodium) • Valpromide • Valnoctamide • Valproate pivoxil
GABA reuptake inhibitor: TiagabineUnknown/multiple/
unsortedPropionatesHypnotics/Sedatives (N05C) GABAA Agonists/PAMs Barbiturates: Allobarbital • Amobarbital • Aprobarbital • Barbital • Butabarbital • Butobarbital • Cyclobarbital • Ethallobarbital • Heptabarbital • Hexobarbital • Mephobarbital • Methohexital • Pentobarbital • Phenobarbital • Proxibarbal • Reposal • Secobarbital • Talbutal • Thiamylal • Thiopental • Vinbarbital • Vinylbital; Benzodiazepines: Brotizolam • Clonazepam • Cinolazepam • Climazolam • Doxefazepam • Estazolam • Flunitrazepam • Flurazepam • Flutoprazepam • Haloxazolam • Loprazolam • Lormetazepam • Midazolam • Nimetazepam • Nitrazepam • Quazepam • Temazepam • Triazolam; Carbamates: Carisoprodol • Ethinamate • Hexapropymate • Meprobamate • Methocarbamol • Procymate • Tybamate; Neuroactive Steroids: Acebrochol • Allopregnanolone • Alphadolone • Alphaxolone • Eltanolone • Ganaxolone • Hydroxydione • Minaxolone • Org 20599 • Org 21465 • Tetrahydrodeoxycorticosterone; Nonbenzodiazepines: CL-218,872 • Eszopiclone • Indiplon • JM-1232 • Lirequinil • Necopidem • Pazinaclone • ROD-188 • Saripidem • Suproclone • Suriclone • SX-3228 • U-89843A • U-90042 • Zaleplon • Zolpidem • Zopiclone; Phenols: Fospropofol • Propofol; Piperidinediones: Glutethimide • Methyprylon • Pyrithyldione • Piperidione; Quinazolinones: Afloqualone • Cloroqualone • Diproqualone • Etaqualone • Mebroqualone • Mecloqualone • Methaqualone • Methylmethaqualone • Nitromethaqualone; Others: 2-Methyl-2-butanol • Acetophenone • Acetylglycinamide chloral hydrate • Bromide (Lithium bromide, Potassium bromide, Sodium bromide) • Centalun • Chloral hydrate • Chloralose • Chloralodol • Clomethiazole • Dichloralphenazone • Ethanol (Alcohol) • Ethchlorvynol • Etomidate • Gaboxadol • Loreclezole • Methylpentynol • Metomidate • Paraldehyde • Petrichloral • Sulfonmethane • Trichloroethanol • Triclofos • Valerenic acid (Valerian)GABAB Agonists H1 Inverse agonists Antihistamines: Captodiame • Cyproheptadine • Dimenhydrinate • Diphenhydramine • Doxylamine • Hydroxyzine • Methapyrilene • Pheniramine • Promethazine • Propiomazine; Others: Tricyclic antidepressants (Amitriptyline, Doxepin, Trimipramine, etc.) • Tetracyclic antidepressants (Mianserin, Mirtazapine, etc.) • Typical antipsychotics (Chlorpromazine, Thioridazine, etc.) • Atypical antipsychotics (Olanzapine, Quetiapine, Risperidone, etc.)α1-Adrenergic Antagonists Mianserin • Niaprazine • Trazodone; Others: Tricyclic antidepressants (Amitriptyline, Doxepin, Trimipramine, etc.) • Typical antipsychotics (Chlorpromazine, Thioridazine, etc.) • Atypical antipsychotics (Olanzapine, Quetiapine, Risperidone, etc.)α2-Adrenergic Agonists 4-NEMD • Clonidine • Detomidine • Dexmedetomidine • Lofexidine • Medetomidine • Romifidine • Tizanidine • Xylazine5-HT2A Antagonists Eplivanserin • Niaprazine • Pruvanserin • Trazodone • Volinanserin; Others: Tricyclic antidepressants (Amitriptyline, Doxepin, Trimipramine, etc.) • Tetracyclic antidepressants (Mianserin, Mirtazapine, etc.) • Typical antipsychotics (Chlorpromazine, Thioridazine, etc.) • Atypical antipsychotics (Olanzapine, Quetiapine, Risperidone, etc.)Melatonin Agonists Orexin Antagonists Almorexant • SB-334,867 • SB-408,124 • SB-649,868 • Suvorexant • TCS-OX2-29Others Acecarbromal • Apronal • Bromisoval • Cannabidiol (Cannabis) • Carbromal • Embutramide • Evoxine • Fenadiazole • Gabapentin • Kavalactones (Kava) • Mephenoxalone • Opiates/Opioids (Hydrocodone, Morphine (Opium), etc.) • Passion flower • Scopolamine (Mandrake) • ValnoctamideCategories:- Barbiturates
- Lethal injection components
- German inventions
Wikimedia Foundation. 2010.
