- Butyl
-
In organic chemistry, butyl is a four-carbon alkyl radical or substituent group with general chemical formula -C4H9, derived from either of the two isomers of butane.
The isomer n-butane can connect either at one of the two terminal carbon atoms or at one of the two internal carbon atoms, giving rise to two "-butyl" groups:
- Normal butyl or n-Butyl: CH3–CH2–CH2–CH2– (fully systematic name: butyl)
- Secondary butyl or sec-Butyl: CH3–CH2–CH(CH3)– (fully systematic name: 1-methylpropyl)
The second, branched isomer of butane, isobutyl, can connect either at one of the three terminal carbons or at the central carbon, giving rise to another two groups:
- Isobutyl: (CH3)2CH–CH2– (fully systematic name: 2-methylpropyl)
- Tertiary butyl, tert-Butyl or t-butyl: (CH3)3C– (fully systematic name: 1,1-dimethylethyl)
Contents
Nomenclature
According to IUPAC nomenclature, "isobutyl", "sec-butyl", and "tert-butyl" are all retained trivial names.
Skeletal formula Common name IUPAC name Systematic name Alternate notation 
n-butyl butyl butyl butan-1-yl 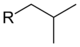
isobutyl isobutyl 2-methylpropyl 2-methylpropan-1-yl 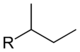
sec-butyl sec-butyl 1-methylpropyl butan-2-yl 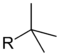
tert-butyl tert-butyl 1,1-dimethylethyl 2-methylpropan-2-yl Butyl is the largest substituent for which trivial names are commonly used for all isomers.
The butyl group's carbon that is connected to the rest (R) of the molecule is called the RI or R-prime carbon[citation needed]. The prefixes sec (from "secondary") and tert (from "tertiary") refer to the number of additional side chains connected to the first butyl carbon. The prefix "iso" (from "isomer") means "equal" while the prefix 'n-' stands for "normal".
Some examples
The following are the four isomers of "butyl acetate":


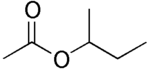
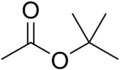
n-butyl acetate isobutyl acetate sec-butyl acetate tert-butyl acetate Etymology
As the number of carbons in an alkyl chain increases, butyl is the last to be named historically instead of through Greek numbers. The name is derived from butyric acid, a four-carbon carboxylic acid found in rancid butter. The name of butyric acid, in turn, comes from Latin butyrum, butter.
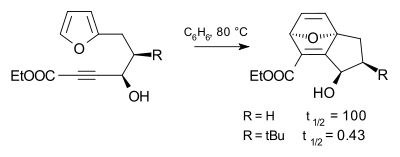
Tert-butyl effect
The tert-butyl substituent is very bulky and used in chemistry for kinetic stabilisation together with other bulky groups such as the related trimethylsilyl group. The effect that the t-butyl group exerts on the progress of a chemical reaction is called the tert-butyl effect.
This effect is illustrated in the Diels-Alder reaction below, where the tert-butyl substituent causes a reaction rate acceleration by a factor of 240 compared to hydrogen as the substituent.[1]
See also
References
- ^ Factors affecting ease of ring formation. The effect of anchoring substitution on the rate of an intramolecular diels-alder reaction with furan-diene Serge Cauwberghs and Pierre J. De Clercq B. Tinant and J. P. Declercq Tetrahedron Letters Volume 29, Issue 20 , 1988, Pages 2493-2496 doi:10.1016/S0040-4039(00)87916-2
Categories:- Alkyl groups
Wikimedia Foundation. 2010.