- Ethosuximide
-
Ethosuximide 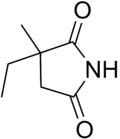
Systematic (IUPAC) name (RS)-3-ethyl-3-methyl-pyrrolidine-2,5-dione Clinical data Trade names Zarontin AHFS/Drugs.com monograph MedlinePlus a682327 Pregnancy cat. D (Australia, United States) Legal status ℞-only (U.S.) Routes Oral Pharmacokinetic data Bioavailability 93%[1] Metabolism Hepatic (CYP3A4, CYP2E1) Half-life 53 hours Excretion Renal (20%) Identifiers CAS number 77-67-8 ATC code N03AD01 PubChem CID 3291 DrugBank APRD00318 ChemSpider 3175 
UNII 5SEH9X1D1D 
KEGG D00539 
ChEBI CHEBI:4887 
ChEMBL CHEMBL696 
Chemical data Formula C7H11NO2 Mol. mass 141.168 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Ethosuximide is a succinimide anticonvulsant, used mainly in absence seizures.
Contents
Uses
Approved
It is approved for absence seizures.[2] Ethosuximide is considered the first choice drug for treating absence seizures in part because it lacks the idiosyncratic hepatotoxicity of the alternative anti-absence drug, valproic acid.[3]
Unapproved
It was reported to have been used for intermittent explosive disorder in 1980 by Drs Andrulonis, Donnelly, Glueck, Stroebel, and Szabek.[4]
Dosage
Therapeutic drug concentrations are individualized according to response and tolerance. Common Serum Therapeutic Range: 40-100 µg/mL. Potentially Toxic Serum Concentration: >100 µg/mL.
Availability
Ethosuximide is marketed under the trade names Emeside and Zarontin. However, both capsule preparations were discontinued from production, leaving only generic preparations available. Emeside capsules were discontinued by their manufacturer, Laboratories for Applied Biology, in 2005.[1] Similarly, Zarontin capsules were discontinued by Pfizer in 2007.[2] Syrup preparations of both brands are still available.
Mechanism of action
There is some controversy over the exact mechanism by which ethosuximide prevents absence seizures. While the view that ethosuximide is a T-type calcium channel blocker gained widespread support following its proposal, attempts to replicate the initial finding were inconsistent.
In March 1989, Coulter, Huguenard and Prince showed that ethosuximide and dimethadione, both effective anti-absence agents, reduced low-threshold Ca2+ currents in T-type Ca2+ channels in freshly removed thalamic neurons.[5] In June of that same year, they also found the mechanism of this reduction to be voltage-dependent, using acutely neurons of rats and guinea pigs; it was also noted that valproic acid, which is also used in absence seizures, did not do that.[6] The next year, they showed that anticonvulsant succinimides did this and that the proconvulsant ones did not.[7] The first part was supported by Kostyuk et al. in 1992, who reported a substantial reduction in current in dorsal root ganglia at concentrations ranging from 7 µmol/L to 1 mmol/L.[8]
That same year, however, Herrington and Lingle found no such effect at concentrations of up to 2.5 mmol/L.[9] The year after, a study conducted on human neocortical cells removed during surgery for intractable epilepsy, the first to use human tissue, found that ethosuximide had no effect on Ca2+ currents at the concentrations typically needed for a therapeutic effect.[10]
In 1998, Slobodan M. Todorovic and Christopher J. Lingle of Washington University reported a 100% block of T-type current in dorsal root ganglia at 23.7 ± 0.5 mmol/L, far higher than Kostyuk reported.[11] That same year, Leresche et al. reported that ethosuximide had no effect on T-type currents, but did decrease noninactivating Na+ current by 60% and the Ca2+-activated K+ currents by 39.1 ± 6.4% in rat and cat thalamocortical cells. It was concluded that the decrease in Na+ current is responsible for the anti-absence properties.[12]
In the introduction of a paper published in 2001, Dr. Juan Carlos Gomora and colleagues at the University of Virginia in Charlottesville pointed out that past studies were often done in isolated neurons that had lost most of their T-type channels.[13] Using cloned α1G, α1H, and α1I T-type calcium channels, Gomora's team found that ethosuximide blocked the channels with an IC50 of 12 ± 2 mmol/L and that of N-desmethylmethsuximide (the active metabolite of mesuximide) is 1.95 ± 0.19 mmol/L for α1G, 1.82 ± 0.16 mmol/L for α1I, and 3.0 ± 0.3 mmol/L for α1H. It was suggested that the blockade of open channels is facilitated by ethosuximide's physically plugging the channels when current flows inward.
Adverse effects
Central nervous system
Common
- suicidal tendencies
- drowsiness
- mental confusion
- insomnia
- nervousness
- headache
- euphoria
- ataxia
- hiccups
- impaired concentration
- irritability
- hyperactivity
- loss of taste
- night terrors
Rare
Gastrointestinal
- dyspepsia
- vomiting
- nausea
- cramps
- constipation
- diarrhea
- stomach pain
- loss of appetite
- weight loss
- gingival hyperplasia
- swelling of tongue
Genitourinary
- microscopic hematuria
- vaginal bleeding
Hematopoietic
The following can occur with or without bone marrow loss:
Integumentary
- urticaria
- systemic lupus erythematosus
- Stevens-Johnson syndrome
- hirsutism
- pruritic erythematous rashes
Ocular
Complications
- abnormal liver function
Drug interactions
Valproates can either decrease or increase the levels of ethosuximide; However, combinations of valproates and ethosuximide had a greater Protective Index than either drug alone.[14]
It may elevate serum phenytoin levels.
Chemistry
Ethosuximide, 3-ethyl-3-methypyrrolidine-2,5-dione is synthesized from methylethylketone and cyanoacetic ester, which undergo a Knoevenagel condensation. Then hydrogen cyanide is added. After acidic hydrolysis and decarboxylation of the synthesized dinitrile, 2-methyl-2-ethylsuccinic acid is formed. Reacting this product with ammonia gives the diammonium salt, and heterocyclization into ethosuximide takes place during subsequent heating.

- L.M. Long, Ch.A. Miller, U.S. Patent 2,993,835 (1961).
- S.S.G. Sircar, J. Chem. Soc., 1252 (1927).
References
Notes
- ^ Patsalos, P. N. (November 2005). "Properties of Antiepileptic Drugs in the Treatment of Idiopathic Generalized Epilepsies". Epilepsia 46 (s9): 140–144. doi:10.1111/j.1528-1167.2005.00326.x. PMID 16302888.
- ^ Pharmaceutical Associates, Incorporated (2000). "Ethosuximide Approval Label" (PDF). Label and Approval History. Food and Drug Administration Center for Drug Evaluation and Research. http://www.fda.gov/cder/foi/anda/2000/40253_Ethosuximide_Prntlbl.pdf. Retrieved 2006-02-05.[dead link]
- ^ "Drugs used in generalized seizures." Katzung, B. Basic and Clinical Pharmacology. 9th Ed. 2003. Lange Medical Books/McGraw-Hill.0071410929.
- ^ Andrulonis, P. A.; J. Donnelly, B. C. Glueck, C. F. Stroebel, and B. L. Szabek (November 1980). "Preliminary data on ethosuximide and the Episodic dyscontrol syndrome". American Journal of Psychiatry 137 (11): 1455–6. PMID 7435689.
- ^ Coulter DA, Huguenard JR, Prince DA. "Specific petit mal anticonvulsants reduce calcium currents in thalamic neurons." Neurosci Lett. 1989 Mar 13;98(1):74-8. PMID 2710401
- ^ "Characterization of ethosuximide reduction of low-threshold calcium current in thalamic neurons." Annals of Neurology. 1989 Jun;25(6):582-93. PMID 2545161
- ^ Coulter DA, Huguenard JR, Prince DA. "Differential effects of petit mal anticonvulsants and convulsants on thalamic neurones: calcium current reduction." British Journal of Pharmacology. 1990 Aug;100(4):800-6. PMID 2169941
- ^ Kostyuk PG, Molokanova EA, Pronchuk NF, Savchenko AN, Verkhratsky AN. "Different action of ethosuximide on low- and high-threshold calcium currents in rat sensory neurons." Neuroscience. 1992 Dec;51(4):755-8. PMID 1336826
- ^ Herrington J, Lingle CJ (July 1992). "Kinetic and pharmacological properties of low voltage-activated Ca2+ current in rat clonal (GH3) pituitary cells". Journal of Neurophysiology 68 (1): 213–32. PMID 1325546. http://jn.physiology.org/cgi/reprint/68/1/213.
- ^ Sayer RJ, Brown AM, Schwindt PC, Crill WE. "Calcium currents in acutely isolated human neocortical neurons." Journal of Neurophysiology. 1993 May;69(5):1596-606. PMID 8389832 Fulltext
- ^ Todorovic SM, Lingle CJ (January 1, 1998). "Pharmacological properties of T-type Ca2+ current in adult rat sensory neurons: effects of anticonvulsant and anesthetic agents". Journal of Neurophysiology 79 (1): 240–52. PMID 9425195. http://jn.physiology.org/cgi/content/full/79/1/240.
- ^ Leresche N, Parri HR, Erdemli G, Guyon A, Turner JP, Williams SR, Asprodini E, Crunelli V (July 1, 1998). "On the action of the anti-absence drug ethosuximide in the rat and cat thalamus". Journal of Neuroscience 18 (13): 4842–53. PMID 9634550. http://www.jneurosci.org/cgi/content/full/18/13/4842.
- ^ Gomora JC, Daud AN, Weiergraber M, Perez-Reyes E (2001). "Block of cloned human T-type calcium channels by succinimide antiepileptic drugs". Molecular Pharmacology 60 (5): 1121–32. PMID 11641441.
- ^ Bourgeois, BF (December 1988). "Combination of valproate and ethosuximide: antiepileptic and neurotoxic interaction". The Journal of Pharmacology and Experimental Therapeutics 247 (3): 1128–32. PMID 3144596.
- ^ "Concern over ethosuximide capsule discontinuation". http://www.pharmj.com/editorial/20051029/news/p539ethosuximide.html. Retrieved 2008-08-31.[dead link]
- ^ "Zarontin capsules discontinued". http://www.epilepsy.org.uk/news/drugwatch/ethosuximide. Retrieved 2008-08-31.
External links
- Ethosuximide Internet Mental Health.
- MedlinePlus Drug Information: Ethosuximide Oral
- Zarontin Pfizer.
- Zarontin Drug information, published studies and current trials
Anticonvulsants (N03) GABAA receptor agonist Clobazam • Clonazepam • Clorazepate • Diazepam# • Flutoprazepam • Lorazepam • Midazolam • Nimetazepam • Nitrazepam • TemazepamOther GABA agents Carbonic anhydrase inhibitor Channel blockers Primarily sodiumPrimarily calciumEthosuximide# • Mesuximide • PhensuximideUnknown/ungroupedChannel openers PotassiumRetigabineIndirect GABA agents GABA transaminase inhibitor: Valproic acid# (Sodium valproate & Valproate semisodium) • Valpromide • Valnoctamide • Valproate pivoxil
GABA reuptake inhibitor: TiagabineUnknown/multiple/
unsortedPropionatesCategories:- Anticonvulsants
- Succinimides
Wikimedia Foundation. 2010.
