- Midazolam
-
Midazolam 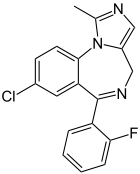
Systematic (IUPAC) name 8-chloro-6-(2-fluorophenyl)-1-methyl-4H-imidazo[1,5-a][1,4]benzodiazepine Clinical data Trade names Dormicum, Hypnovel, Versed AHFS/Drugs.com monograph MedlinePlus a609003 Pregnancy cat. D (USA)
C (Aus)Legal status Schedule IV(US) Routes Oral, I.M., I.V., parenteral Pharmacokinetic data Bioavailability Oral ~36%
I.M. 90%+Protein binding 97% Metabolism Hepatic Half-life 1.8-6.4 hours Excretion Renal Identifiers CAS number 59467-70-8 
ATC code N05CD08 PubChem CID 4192 DrugBank APRD00680 ChemSpider 4047 
UNII R60L0SM5BC 
KEGG D00550 
ChEMBL CHEMBL655 
Chemical data Formula C18H13ClFN3 Mol. mass 325.78 SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Midazolam (
 /mɪˈdæzəlæm/; marketed in English-speaking countries under the trade names Dormicum,[1] Hypnovel,[2] and Versed[3]) is a short-acting drug in the benzodiazepine class developed by Hoffmann-La Roche in the 1970s[4]. The drug is used for treatment of acute seizures, moderate to severe insomnia, and for inducing sedation and amnesia before medical procedures. It possesses profoundly potent anxiolytic, amnestic, hypnotic, anticonvulsant, skeletal muscle relaxant, and sedative properties.[5][6][7] Midazolam has a fast recovery time and is the most commonly used benzodiazepine as a premedication for sedation; less commonly it is used for induction and maintenance of anesthesia. Flumazenil is a benzodiazepine antagonist drug that can be used to treat an overdose of midazolam as well as to reverse sedation.[6] However, flumazenil can trigger seizures in mixed overdoses and in benzodiazepine dependent individuals so is not used in most cases.[8][9]
/mɪˈdæzəlæm/; marketed in English-speaking countries under the trade names Dormicum,[1] Hypnovel,[2] and Versed[3]) is a short-acting drug in the benzodiazepine class developed by Hoffmann-La Roche in the 1970s[4]. The drug is used for treatment of acute seizures, moderate to severe insomnia, and for inducing sedation and amnesia before medical procedures. It possesses profoundly potent anxiolytic, amnestic, hypnotic, anticonvulsant, skeletal muscle relaxant, and sedative properties.[5][6][7] Midazolam has a fast recovery time and is the most commonly used benzodiazepine as a premedication for sedation; less commonly it is used for induction and maintenance of anesthesia. Flumazenil is a benzodiazepine antagonist drug that can be used to treat an overdose of midazolam as well as to reverse sedation.[6] However, flumazenil can trigger seizures in mixed overdoses and in benzodiazepine dependent individuals so is not used in most cases.[8][9]Administration of midazolam by nose or the buccal route (absorption via the gums and cheek) as an alternative to rectally administered diazepam is becoming increasingly popular for the emergency treatment of seizures in children.[10] Midazolam is also used for endoscopy[11] procedural sedation and sedation in intensive care.[12][13] The anterograde amnesia property of midazolam is useful for premedication before surgery to inhibit unpleasant memories.[14] Midazolam, like many other benzodiazepines, has a rapid onset of action, high effectiveness and low toxicity level. Drawbacks of midazolam include drug interactions, tolerance, withdrawal syndrome as well as adverse events including cognitive impairment and sedation.[14] Paradoxical effects occasionally occur and are most common in children, the elderly,[14] and particularly after intravenous administration.[15]
Contents
Indications
Intravenous midazolam is indicated for procedural sedation (often in combination with an opioid, such as fentanyl), for preoperative sedation, for the induction of general anesthesia, and for sedation of ventilated patients in critical care units.[12][13] Midazolam is superior to diazepam in impairing memory of endoscopy procedures, but propofol has a quicker recovery time and a better memory-impairing effect.[11] It is the most popular benzodiazepine in the intensive care unit (ICU) because of its short elimination half-life, combined with its water solubility and its suitability for continuous infusion. However, for long-term sedation, lorazepam is preferred due to its long duration of action,[16] and propofol has advantages over midazolam when used in the ICU for sedation, such as shorter weaning time and earlier tracheal extubation.[17] There is evidence buccal and intranasal midazolam is easier to administer and more effective than rectally administered diazepam in the emergency control of seizures.[18][19][20] In the final stages of end-of-life care, midazolam is routinely used at low doses via subcutaneous injection to alleviate agitation, myoclonus, restlessness or anxiety in the last hours or days of life.[21] At higher doses during the last weeks of life, midazolam is considered a first line agent in palliative continuous deep sedation therapy when it is necessary to alleviate intolerable suffering unresponsive to other treatments,[22] but the need for this is more rare.[23] Midazolam is also sometimes used in neonates who are receiving mechanical ventilation, although morphine is preferred, owing to its better safety profile for this indication.[24]
Oral midazolam is indicated for the short term treatment of moderately severe insomnia in patients who have not reacted adequately to other hypnotics, and who have persistent trouble in falling asleep. Because of midazolam's extremely short duration, it is not used for patients who have trouble staying asleep through the night; moderate to long acting benzodiazepines, such as temazepam, nitrazepam, flunitrazepam and lormetazepam, are used for those purposes. Like other benzodiazepines, midazolam produces a decrease in delta activity, though the effect of benzodiazepines on delta may not be mediated via benzodiazepine receptors. Delta activity is an indicator of depth of sleep within non-REM sleep; it is thought to reflect sleep quality, with lower levels of delta sleep reflecting poorer sleep. Thus, midazolam and other benzodiazepines cause a deterioration in sleep quality. Cyproheptadine may be superior to nitrazepam in the treatment of insomnia, as it enhances sleep quality based on EEG studies.[25]
Midazolam in combination with an antipsychotic drug is indicated for the acute management of schizophrenia when it is associated with aggressive or out of control behaviour.[26] It is also is sometimes used for the acute management of seizures such as status epilepticus. Long-term use for the management of epilepsy is not recommended, however, due to the significant risk of tolerance (which renders midazolam and other benzodiazepines ineffective) and the significant side effect of sedation.[27] A benefit of midazolam is that in children it can be administered buccally or intranasally at home or at school for emergency control of acute seizures, including status epilepticus.[10][28] Midazolam is effective for refractory status epilepticus, and has advantages of being water soluble, having a rapid onset of action and not causing metabolic acidosis from the propylene glycol vehicle, which occurs with other benzodiazepines. Drawbacks include a high degree of breakthrough seizures—due to the short half-life of midazolam—in over 50 percent of people treated, as well as treatment failure in 14–18 percent of people with refractory status epilepticus. Tolerance develops rapidly to the anticonvulsant effect, and the dose may need to be increased by several times to maintain anticonvulsant therapeutic effects. With prolonged use, tolerance and tachyphylaxis can occur and the elimination half-life may increase, up to days.[14][29]
Availability
Dormicum brand midazolam is marketed by Roche as white, oval 7.5 mg tablets in boxes of 2 or 3 blisterstrips of 10 tablets, and as blue, oval 15 mg tablets in boxes of 2 blisterstrips of 10 tablets. The tablets are imprinted with "Roche" on one side and the dose of the tablet on the other side. Dormicum is also available as 1 ml, 3 ml and 10 ml ampoules at a concentration of 5 mg/ml. Another manufacturer, Novell Pharmaceutical Laboratories, makes it available as Miloz in 3 ml and 5 ml ampoules. Midazolam is the only water-soluble benzodiazepine available. Another maker of the product is Roxanne Laboratories in an oral solution Midazolam HCl Syrup, 2mg/mL Clear, red to purplish-red syrup and is cherry in flavor. It becomes soluble when the injectable solution is buffered to a pH of 2.9–3.7. Midazolam is also available in liquid form.[14] Midazolam can be administered intramuscularly,[28] intravenously,[30] intrathecally,[31] intranasally,[19] buccally,[32] or orally.[14]
Pregnancy
Midazolam, when taken during the third trimester of pregnancy, may cause severe risk to the neonate, including benzodiazepine withdrawal syndrome with possible symptoms including hypotonia, apnoeic spells, cyanosis, and impaired metabolic responses to cold stress. Symptoms of hypotonia and the neonatal benzodiazepine withdrawal syndrome have been reported to persist from hours to months after birth.[33] Other neonatal withdrawal symptoms include hyperexcitability, tremor and gastrointestinal upset (diarrhea or vomiting). Breast feeding by mothers using midazolam is not recommended.[34]
Neonates
Midazolam is sometimes used in neonatal intensive care unit care. When used, additional caution is required in neonates; midazolam should not be used for longer than 72 hours due to risks of tachyphylaxis, and the possibility of development of a benzodiazepine withdrawal syndrome, as well as neurological complications. Bolus injections should be avoided due to the increased risk of cardiovascular depression, as well as neurological complications.[35]
Children
In 2011, the European Medicines Agency granted a marketing authorisation for a buccal application form of midazolam, sold under the trade name Buccolam. Buccolam was approved for the treatment of prolonged, acute, convulsive seizures in patients from three months to less than 18 years of age. This was the first application of a paediatric-use marketing authorisation (PUMA).[36][37]
Elderly
Additional caution is required in the elderly, as they are more sensitive to the pharmacological effects of benzodiazepines and also metabolise them more slowly, and are more prone to adverse effects, including drowsiness, amnesia (especially anterograde amnesia), ataxia, hangover effects, confusion and falls.[14]
Contraindications
Benzodiazepines require special precaution if used in the elderly, during pregnancy, in children, in alcohol or drug-dependent individuals or individuals with comorbid psychiatric disorders.[38] Additional caution is required in critically ill patients, as accumulation of midazolam and its active metabolites may occur.[39] Kidney or liver impairments may slow down the elimination of midazolam leading to prolonged and enhanced effects.[6][40] Contraindications include hypersensitivity, acute narrow angle glaucoma, shock, hypotension or head injury. Most are relative contraindications.
Side effects
Side effects of midazolam in the elderly are listed above.[14] People experiencing amnesia as a side effect of midazolam are generally unaware their memory is impaired.[41]
Long-term use of benzodiazepines has been associated with long-lasting deficits of memory, and show only partial recovery six months after stopping benzodiazepines.[14] It is unclear whether full recovery occurs after longer periods of abstinence. Benzodiazepines can cause or worsen depression.[14] Paradoxical excitement occasionally occurs with benzodiazepines, including a worsening of seizures. Children and elderly individuals or those with a history of alcohol abuse and individuals with a history of aggressive behavior or anger are at increased risk of paradoxical effects.[14] Paradoxical reactions are particularly associated with intravenous administration.[15] After nighttime administration of midazolam, residual 'hangover' effects, such as sleepiness and impaired psychomotor and cognitive functions, may persist into the next day. This may impair the ability of users to drive safely and may increase the risk of falls and hip fractures.[42] Sedation, respiratory depression and hypotension due to a reduction in systematic vascular resistance, and an increase in heart rate can occur.[6][28] If IV midazolam is given too quickly, hypotension may occur. A “midazolam infusion syndrome” may result from high doses, and is characterised by delayed arousal hours to days after discontinuation of midazolam, and may lead to an increase in the length of ventilatory support needed.[43]
In susceptible individuals, midazolam has been known to cause a paradoxical reaction, a well-documented complication with benzodiazapines. When this occurs, the individual may experience anxiety, involuntary movements, aggressive or violent behavior, uncontrollable crying or verbalization, and other similar effects. This seems to be related to the altered state of consciousness or disinhibition produced by the drug. Paradoxical behavior is often not recalled by the patient due to the amnesia-producing properties of the drug. In extreme situations, flumazenil can be administered to inhibit or reverse the effects of midazolam. Antipsychotic medications, such as haloperidol, have also been used for this purpose.[44]
Midazolam is known to cause respiratory depression. In healthy humans, 0.15 mg/kg of midazolam may cause respiratory depression, which is postulated to be a CNS effect.[45] When midazolam is administered in combination with fentanyl, the incidence of hypoxemia or apnea becomes more likely.[46]
Tolerance, dependence and withdrawal
A benzodiazepine dependence occurs in about one third of individuals who are treated with benzodiazepines for longer than 4 weeks,[14] which typically results in tolerance and benzodiazepine withdrawal syndrome when the dose is reduced too rapidly. Midazolam infusions may induce tolerance and a withdrawal syndrome in a matter of days. The risk factors for dependence include dependent personality, use of a benzodiazepine which is short-acting, high potency and long-term use of benzodiazepines. Withdrawal symptoms from midazolam can range from insomnia and anxiety to seizures and psychosis. Withdrawal symptoms can sometimes resemble a persons underlying condition. Gradual reduction of midazolam after regular use can minimise withdrawal and rebound effects. Tolerance and the resultant withdrawal syndrome may be due to receptor down-regulation and GABAA receptor alterations in gene expression which results in long-term changes in the function of the GABAergic neuronal system.[14][47][48]
Chronic users of benzodiazepine medication who are given midazolam experience reduced therapeutic effects of midazolam, due to tolerance to benzodiazepines.[43][49] Prolonged infusions with midazolam results in the development of tolerance; if midazolam is given for a few days or more a withdrawal syndrome can occur. Therefore in order to prevent a withdrawal syndrome a prolonged infusion needs to be gradually withdrawn and sometimes if necessary continued tapering of dose with an oral long-acting benzodiazepine such as clorazepate dipotassium. When signs of tolerance to midazolam occur during intensive care unit sedation the addition of an opioid or propofol is recommended. Withdrawal symptoms can include irritability, abnormal reflexes, tremors, clonus, hypertonicity, delirium, and seizures, nausea, vomiting and diarrhea, tachycardia, hypertension and tachypnea.[43]
Overdose
See also: Benzodiazepine overdoseA midazolam overdose is considered a medical emergency and generally requires the immediate attention of medical personnel. Benzodiazepine overdose in healthy individuals is rarely life threatening; however, the toxicity of benzodiazepines increases when they are combined with other CNS depressants such as alcohol, opioids, or tricyclic antidepressants. The toxicity of benzodiazepine overdose and risk of death is also increased in the elderly and those with obstructive pulmonary disease or when used intravenously. Treatment is supportive; activated charcoal can be used within an hour of the overdose. The antidote for an overdose of midazolam (or any other benzodiazepine) is flumazenil (Anexate).[6] While effective in reversing the effects of benzodiazepines it is not used in most cases as it may trigger seizures in mixed overdoses and benzodiazepine dependent individuals.[8][9]
Symptoms of midazolam overdose can include:[8][9]
- Ataxia
- Dysarthria
- Nystagmus
- Slurred speech
- Somnolence (difficulty staying awake)
- Mental confusion
- Hypotension
- Respiratory arrest
- Vasomotor collapse
Detection in body fluids
The concentrations of midazolam and/or its major metabolite, 1-hydroxymidazolam glucuronide, may be quantified in plasma, serum or whole blood in order to monitor for safety in those receiving the drug therapeutically, to confirm a diagnosis of poisoning in hospitalized patients or to assist in a forensic investigation of a case of fatal overdosage. Patients with renal dysfunction may exhibit prolongation of elimination half-life for both the parent drug and its active metabolite, with accumulation of these two substances in the bloodstream and the appearance of adverse depressant effects.[50]
Interactions
HIV protease inhibitors, nefazodone, sertraline, grapefruit juice, fluoxetine, erythromycin, diltiazem, clarithromycin inhibit the metabolism of midazolam, leading to a prolonged action. St John's wort, rifapentine, rifampin, rifabutin, phenytoin enhance the metabolism of midazolam leading to a reduced action. Sedating antidepressants, antiepileptic drugs such as phenobarbital, phenytoin and carbamazepine, sedative antihistamines, opiates, antipsychotics and alcohol enhance the sedative effects of midazolam.[14] Midazolam is metabolized almost completely by cytochrome P450-3A4. Atorvastatin administration along with midazolam results in a reduced elimination rate of midazolam.[51] St John's wort decreases the blood levels of midazolam.[52] Grapefruit juice reduces intestinal 3A4 and results in less metabolism and higher plasma concentrations.[53]
Pharmacology
Midazolam is a short-acting benzodiazepine in adults with an elimination half-life of one to four hours; however, in the elderly, as well as young children and adolescents, the elimination half-life is longer.[7][15] Midazolam is metabolised into an active metabolite alpha1-hydroxymidazolam. Age related deficits, renal and liver status affect the pharmacokinetic factors of midazolam as well as its active metabolite.[54] However, the active metabolite of midazolam is minor and contributes to only 10 percent of biological activity of midazolam. Midazolam is poorly absorbed orally with only 50 percent of the drug reaching the bloodstream.[14] Midazolam is metabolised by cytochrome P450 (CYP) enzymes and by glucuronide conjugation. The therapeutic as well as adverse effects of midazolam are due to its effects on the GABAA receptors; midazolam does not activate GABAA receptors directly but, as with other benzodiazepines, it enhances the effect of the neurotransmitter GABA on the GABAA receptors (↑ frequency of Cl− channel opening) resulting in neural inhibition. Almost all of the properties can be explained by the actions of benzodiazepines on GABAA receptors. This results in the following pharmacological properties being produced: sedation, hypnotic, anxiolytic, anterograde amnesia, muscle relaxation and anti-convulsant.[6]
History
Midazolam is among about 35 benzodiazepines which are currently used medically,[55] and was synthesised in 1975 by Walser and Fryer at Hoffmann-LaRoche, Inc in the United States.[56] Owing to its water solubility, it was found to be less likely to cause thrombophlebitis than similar drugs.[57][58] The benzodiazepine drug alprazolam was synthesised in 1976 from midazolam[59][60] and introduced to the United States market in 1981.[61] The anticonvulsant properties of midazolam were studied in the late 1970s, but not until the 1990s did it emerge as an effective treatment for convulsive status epilepticus.[62] As of 2010[update], it is the most commonly used benzodiazepine in anesthetic medicine.[63] In acute medicine, midazolam has become more popular than other benzodiazepines, such as lorazepam and diazepam, because it is shorter lasting, is more potent, and causes less pain at the injection site.[55] Midazolam is also becoming increasingly popular in veterinary medicine due to its water solubility.[64]
Legal status
In the Netherlands, midazolam is a List II drug of the Opium Law. Midazolam is a Schedule IV drug under the Convention on Psychotropic Substances.[65] In the United Kingdom midazolam is a Class C controlled drug.[66]
See also
- Long term effects of benzodiazepines
- Dexmedetomidine
References
- ^ Roche Products. "Dormicum Package Insert" (PDF). South Africa: Analgo-sedation anesthetist. http://www.masc.co.za/dormicumpackage.htm.
- ^ Hoffman-La Roche (26 October 2007). "HYPNOVEL (midazolam)" (PDF). Australia: Hoffman-La Roche. http://www.roche-australia.com/downloads/hypnovel-pi.cfm?action=get.
- ^ The American Society of Health-System Pharmacists, Inc (1 March 2009). "Midazolam Injection". USA: National Institutes of Health. http://www.nlm.nih.gov/medlineplus/druginfo/meds/a609014.html.
- ^ US Patent 4280957 - Imidazodiazepines and processes therefor
- ^ Mandrioli R, Mercolini L, Raggi MA (October 2008). "Benzodiazepine metabolism: an analytical perspective". Curr. Drug Metab. 9 (8): 827–44. doi:10.2174/138920008786049258. PMID 18855614. http://www.benthamdirect.org/pages/content.php?CDM/2008/00000009/00000008/0009F.SGM.
- ^ a b c d e f Olkkola, KT.; Ahonen, J. (2008). "Midazolam and other benzodiazepines.". Handb Exp Pharmacol 182 (182): 335–60. doi:10.1007/978-3-540-74806-9_16. PMID 18175099.
- ^ a b Barash, Paul G.; Cullen, Bruce F.; Stoelting, Robert K.; Cahalan, Michael D. (1 April 2009). Clinical Anesthesia (6 ed.). Lippincott Williams Wilkins. p. 588. ISBN 978-0-7817-8763-5. http://books.google.com/?id=-YI9P2DLe9UC&pg=PA588.
- ^ a b c A. Boon, Nicholas; Davidson, Stanley; R. Colledge, Nicki; Walker, Brian; Hunter, John G. (2006). Davidson's principles practice of medicine. Edinburgh: Elsevier/Churchill Livingstone. pp. 212–213. ISBN 978-0-443-10057-4. http://books.google.com/?id=TI8DHMKDF-4C&pg=PA212.
- ^ a b c A Rastegar, Darius; I Fingerhood, Michael (2005). Addiction medicine: an evidence-based handbook. Philadelphia, PA: Lippincott Williams Wilkins. p. 80. ISBN 978-0-7817-6154-3. http://books.google.com/?id=DKUQ5YrFZd8C&pg=PA80.
- ^ a b Appleton, R.; Macleod, S.; Martland, T.; Appleton, Richard (July 16, 2008). "Drug management for acute tonic-clonic convulsions including convulsive status epilepticus in children." (PDF). Cochrane Database Syst Rev (3): CD001905. doi:10.1002/14651858.CD001905.pub2. PMID 18646081. http://mrw.interscience.wiley.com/cochrane/clsysrev/articles/CD001905/pdf_fs.html.
- ^ a b McQuaid, KR.; Laine, L. (May 2008). "A systematic review and meta-analysis of randomized, controlled trials of moderate sedation for routine endoscopic procedures.". Gastrointest Endosc 67 (6): 910–23. doi:10.1016/j.gie.2007.12.046. PMID 18440381.
- ^ a b Brown, TB.; Lovato, LM.; Parker, D. (Jan 2005). "Procedural sedation in the acute care setting.". Am Fam Physician 71 (1): 85–90. PMID 15663030.
- ^ a b O'Connor, M.; Bucknall, T.; Manias, E. (2009-09-21). "Sedation Management in Australian and New Zealand Intensive Care Units: Doctors' and Nurses' Practices and Opinions.". Am J Crit Care 19 (3): 285–95. doi:10.4037/ajcc2009541. PMID 19770414.
- ^ a b c d e f g h i j k l m n o Riss, J.; Cloyd, J.; Gates, J.; Collins, S. (Aug 2008). "Benzodiazepines in epilepsy: pharmacology and pharmacokinetics.". Acta Neurol Scand 118 (2): 69–86. doi:10.1111/j.1600-0404.2008.01004.x. PMID 18384456. http://www3.interscience.wiley.com/cgi-bin/fulltext/120119477/HTMLSTART.
- ^ a b c Rosenbaum, A.; Kain, ZN.; Larsson, P.; Lönnqvist, PA.; Wolf, AR. (Sep 2009). "The place of premedication in pediatric practice." (PDF). Paediatr Anaesth 19 (9): 817–28. doi:10.1111/j.1460-9592.2009.03114.x. PMID 19691689. http://www3.interscience.wiley.com/cgi-bin/fulltext/122539081/PDFSTART.
- ^ Arcangeli, A.; Antonelli, M.; Mignani, V.; Sandroni, C. (Nov 2005). "Sedation in PACU: the role of benzodiazepines.". Curr Drug Targets 6 (7): 745–8. doi:10.2174/138945005774574416. PMID 16305452.
- ^ De Cosmo, G.; Congedo, E.; Clemente, A.; Aceto, P. (Nov 2005). "Sedation in PACU: the role of propofol.". Curr Drug Targets 6 (7): 741–4. doi:10.2174/138945005774574425. PMID 16305451.
- ^ Eriksson, K.; Kälviäinen, R. (Nov 2005). "Pharmacologic management of convulsive status epilepticus in childhood.". Expert Rev Neurother 5 (6): 777–83. doi:10.1586/14737175.5.6.777. PMID 16274335.
- ^ a b Wolfe, TR.; Macfarlane, TC. (May 2006). "Intranasal midazolam therapy for pediatric status epilepticus.". Am J Emerg Med 24 (3): 343–6. doi:10.1016/j.ajem.2005.11.004. PMID 16635708.
- ^ Sofou, K.; Kristjánsdóttir, R.; Papachatzakis, NE.; Ahmadzadeh, A.; Uvebrant, P. (Aug 2009). "Management of prolonged seizures and status epilepticus in childhood: a systematic review.". J Child Neurol 24 (8): 918–26. doi:10.1177/0883073809332768. PMID 19332572.
- ^ Liverpool Care Pathway (January 2005). "Care of the Dying Pathway (lcp) (Hospital)" (PDF). United Kingdom. http://www.mcpcil.org.uk/liverpool-care-pathway/pdfs/LCP%20HOSPITAL%20VERSION%2011%20%28printable%20version%29.pdf.
- ^ de Graeff, A.; Dean, M. (Feb 2007). "Palliative sedation therapy in the last weeks of life: a literature review and recommendations for standards.". J Palliat Med 10 (1): 67–85. doi:10.1089/jpm.2006.0139. PMID 17298256.
- ^ Royal College of Physicians (September 2009). "National care of the dying audit 2009". United Kingdom. http://www.rcplondon.ac.uk/media/press-releases/Pages/National-care-of-the-dying-audit-2009.aspx. "[I]n their last 24 hours... 31% had low doses of medication to [control distress from agitation or restlessness]... the remaining 4% required higher doses"
- ^ Bellù, R.; de Waal, KA.; Zanini, R.; Bellù, Roberto (2008). "Opioids for neonates receiving mechanical ventilation.". Cochrane Database Syst Rev (1): CD004212. doi:10.1002/14651858.CD004212.pub3. PMID 18254040.
- ^ Tokunaga S; Takeda Y, Shinomiya K, Hirase M, Kamei C. (February 2007). "Effects of some H1-antagonists on the sleep-wake cycle in sleep-disturbed rats" (PDF). J Pharmacol Sci. 103 (2): 201–6. doi:10.1254/jphs.FP0061173. PMID 17287588. http://www.jstage.jst.go.jp/article/jphs/103/2/201/_pdf.
- ^ Huf, G.; Alexander, J.; Allen, MH.; Raveendran, NS.; Huf, Gisele (2009). "Haloperidol plus promethazine for psychosis-induced aggression." (PDF). Cochrane Database Syst Rev (3): CD005146. doi:10.1002/14651858.CD005146.pub2. PMID 19588366. http://mrw.interscience.wiley.com/cochrane/clsysrev/articles/CD005146/pdf_fs.html.
- ^ Isojärvi, JI; Tokola RA. (December 1998). "Benzodiazepines in the treatment of epilepsy in people with intellectual disability". J Intellect Disabil Res. 42 (1): 80–92. PMID 10030438.
- ^ a b c Walker, M. (Sep 2005). "Status epilepticus: an evidence based guide.". BMJ 331 (7518): 673–7. doi:10.1136/bmj.331.7518.673. PMC 1226249. PMID 16179702. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1226249.
- ^ Murthy, JM. (Dec 2006). "Refractory status epilepticus.". Neurol India 54 (4): 354–8. doi:10.4103/0028-3886.28104. PMID 17114841. http://www.neurologyindia.com/article.asp?issn=0028-3886;year=2006;volume=54;issue=4;spage=354;epage=358;aulast=Murthy.
- ^ Steib, A.; Hausberger, D.; Robillart, A.; Roche, A.; Franckhauser, D.; Dupeyron, JP. (Jun 2006). "[Anaesthetic considerations for interventional radiology]". Ann Fr Anesth Reanim 25 (6): 615–25. doi:10.1016/j.annfar.2006.01.018. PMID 16632296.
- ^ Ho, KM.; Ismail, H. (May 2008). "Use of intrathecal midazolam to improve perioperative analgesia: a meta-analysis.". Anaesth Intensive Care 36 (3): 365–73. PMID 18564797.
- ^ Beran, RG. (Apr 2008). "An alternative perspective on the management of status epilepticus.". Epilepsy Behav 12 (3): 349–53. doi:10.1016/j.yebeh.2007.12.013. PMID 18262847.
- ^ McElhatton PR. (Nov-Dec 1994). "The effects of benzodiazepine use during pregnancy and lactation". Reprod Toxicol. 8 (6): 461–75. doi:10.1016/0890-6238(94)90029-9. PMID 7881198.
- ^ Serreau, R.; Collége national des gynécologues et obstétriciens; Société française de médecine périnatale; Société française de néonatalogie; Société française de anesthésie et de réanimation (Apr 2010). "[Drugs during preeclampsia. Fetal risks and pharmacology]". Ann Fr Anesth Reanim 29 (4): e37–46. doi:10.1016/j.annfar.2010.02.016. PMID 20347563.
- ^ Rawicz, M. (Oct-Dec 2008). "[Recommendations for analgesia and sedation in neonatal intensive care]". Med Wieku Rozwoj 12 (4 Pt 1): 958–67. PMID 19471072.
- ^ "Monthly Report". Committee for Medicinal Products for Human Use (CHMP). 5 July 2011. p. 1. http://www.ema.europa.eu/docs/en_GB/document_library/Committee_meeting_report/2011/07/WC500108232.pdf.
- ^ PR Newswire (6 September 2011). "ViroPharma's Buccolam (Midazolam, Oromucosal Solution) Granted European Marketing Authorization for Treatment of Acute Seizures". http://www.wallstreet-online.de/nachricht/3364595-viropharma-s-buccolam-midazolam-oromucosal-solution-granted-european-marketing-authorization-for-treatment-of-acute-seizures.
- ^ Authier, N.; Balayssac, D.; Sautereau, M.; Zangarelli, A.; Courty, P.; Somogyi, AA.; Vennat, B.; Llorca, PM. et al. (Nov 2009). "Benzodiazepine dependence: focus on withdrawal syndrome.". Ann Pharm Fr 67 (6): 408–13. doi:10.1016/j.pharma.2009.07.001. PMID 19900604.
- ^ Cox, CE.; Reed, SD.; Govert, JA.; Rodgers, JE.; Campbell-Bright, S.; Kress, JP.; Carson, SS. (Mar 2008). "Economic evaluation of propofol and lorazepam for critically ill patients undergoing mechanical ventilation.". Crit Care Med 36 (3): 706–14. doi:10.1097/CCM.0B013E3181544248. PMC 2763279. PMID 18176312. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2763279.
- ^ Verbeeck, RK. (Dec 2008). "Pharmacokinetics and dosage adjustment in patients with hepatic dysfunction." (PDF). Eur J Clin Pharmacol 64 (12): 1147–61. doi:10.1007/s00228-008-0553-z. PMID 18762933. http://www.springerlink.com/content/t56l04u3w07wj031/fulltext.pdf.
- ^ Merritt P, Hirshman E, Hsu J, Berrigan M (2005). "Metamemory without the memory: are people aware of midazolam-induced amnesia?". Psychopharmacology (Berl) 177 (3): 336–43. doi:10.1007/s00213-004-1958-8. PMID 15290003.
- ^ Vermeeren A. (2004). "Residual effects of hypnotics: epidemiology and clinical implications". CNS Drugs. 18 (5): 297–328. doi:10.2165/00023210-200418050-00003. PMID 15089115.
- ^ a b c Mencía, SB.; López-Herce, JC.; Freddi, N. (May 2007). "Analgesia and sedation in children: practical approach for the most frequent situations.". J Pediatr (Rio J) 83 (2 Suppl): S71–82. doi:10.2223/JPED.1625. PMID 17530139. http://www.jped.com.br/conteudo/07-83-S71/ing.asp?cod=1625.
- ^ Carissa E. Mancuso, Maria G. Tanzi, Michael Gabay (2004). "Paradoxical Reactions to Benzodiazepines: Midazolam". Pharmacotherapy. http://www.medscape.com/viewarticle/489358_6.
- ^ Reves JG, Fragen RJ, Vinik HR, Greenblatt DJ (1985). "Midazolam: Pharmacology and Uses". Anesthesiology 62 (3): 310–24. PMID 3156545. http://journals.lww.com/anesthesiology/Citation/1985/03000/Midazolam__Pharmacology_and_Uses.17.aspx.
- ^ Bailey PL, Pace NL, Ashburn MA, Moll JW, East KA, Stanley TH (1990). "Frequent hypoxemia and apnea after sedation with midazolam and fentanyl". Anesthesiology 73 (5): 826–30. PMID 2122773. http://www.ncbi.nlm.nih.gov/pubmed/2122773.
- ^ Fukuda K, Shoda T, Mima H, Uga H (August 2002). "Midazolam induces expression of c-Fos and EGR-1 by a non-GABAergic mechanism". Anesth. Analg. 95 (2): 373–8, table of contents. doi:10.1097/00000539-200208000-00024. PMID 12145054. http://www.anesthesia-analgesia.org/cgi/pmidlookup?view=long&pmid=12145054.
- ^ Cho HH, O'Connell JP, Cooney MF, Inchiosa MA (2007). "Minimizing tolerance and withdrawal to prolonged pediatric sedation: case report and review of the literature". J Intensive Care Med 22 (3): 173–9. doi:10.1177/0885066607299556. PMID 17569173. http://jic.sagepub.com/cgi/pmidlookup?view=long&pmid=17569173.
- ^ Potokar J, Coupland N, Wilson S, Rich A, Nutt D (September 1999). "Assessment of GABA(A)benzodiazepine receptor (GBzR) sensitivity in patients on benzodiazepines". Psychopharmacology (Berl.) 146 (2): 180–4. doi:10.1007/s002130051104. PMID 10525753. http://link.springer.de/link/service/journals/00213/bibs/9146002/91460180.htm.
- ^ Baselt, Randall C (2008). Disposition of Toxic Drugs and Chemicals in Man (8th ed.). Foster City CA: Biomedical Publications. pp. 1037–40. ISBN 978-0-9626523-7-0.
- ^ Mc Donnell, CG; Harte, S; O'driscoll, J; O'loughlin, C; Van Pelt, FN; Shorten, GD (2003). "The effects of concurrent atorvastatin therapy on the pharmacokinetics of intravenous midazolam.". Anaesthesia 58 (9): 899–904. doi:10.1046/j.1365-2044.2003.03339.x. PMID 12911366.
- ^ Hu, Z.; Yang, X.; Ho, PC.; Chan, SY.; Heng, PW.; Chan, E.; Duan, W.; Koh, HL. et al. (2005). "Herb-drug interactions: a literature review.". Drugs 65 (9): 1239–82. doi:10.2165/00003495-200565090-00005. PMID 15916450.
- ^ Arayne, MS.; Sultana, N.; Bibi, Z. (Oct 2005). "Grape fruit juice-drug interactions.". Pak J Pharm Sci 18 (4): 45–57. PMID 16380358.
- ^ Spina, SP.; Ensom, MH. (Mar 2007). "Clinical pharmacokinetic monitoring of midazolam in critically ill patients.". Pharmacotherapy 27 (3): 389–98. doi:10.1592/phco.27.3.389. PMID 17316150.
- ^ a b Udaykumar, Padmaja (30 May 2008). Short Textbook of Pharmacology for Dental and Allied Health Sciences. Jaypee Brothers Medical Publishers. p. 128. ISBN 978-81-8448-149-5. http://books.google.com/?id=L1DgbIE0V9sC.
- ^ Armin Walser, Rodney I. Fryer, Louis Benjamin. Imidazo[1,5-.alpha.][1,4]benzodiazepines. US Patent 4166185, issued to Hoffmann-LaRoche Aug 28, 1979.
- ^ Kaplan, Joel H.; Reich, David L.; Lake, Carol L.; Konstadt, Steven N. (15 May 2006). Cardiac Anesthesia (5 ed.). W.B. Saunders Company. ISBN 978-1-4160-0253-6.
- ^ Malamed, Stanley F. (16 October 2002). Sedation: a guide to patient management. St. Louis: Mosby. p. 335. ISBN 978-0-323-01226-3. http://books.google.com/?id=StZpAAAAMAAJ.
- ^ Vardanyan, Ruben; Vardanyan, ۊRuben; Hruby, Victor J. (10 March 2006). Synthesis of essential drugs. Amsterdam: Elsevier. p. 7. ISBN 978-0-444-52166-8. http://books.google.com/?id=Jjc7KYWZdOYC.
- ^ Jackson B.; Hester, Jr. (19 October 1976). "6-Phenyl-4H-s-triazolo[4,3-a[1,4]benzodiazepines"]. United States Patent and Trademark Office. http://www.google.com/patents?vid=3987052.
- ^ Walker, Sydney (3 December 1996). A dose of sanity: mind, medicine, and misdiagnosis. New York: John Wiley & Sons. pp. 64–65. ISBN 978-0-471-19262-6. http://books.google.com/?id=1H_jx9XO4dcC&pg=PA65.
- ^ S. Wheeler, Derek; R. Wong, Hector; P. Shanley, Thomas (2007). Pediatric critical care medicine: basic science and clinical evidence. London: Springer. p. 984. ISBN 978-1-84628-463-2. http://books.google.com/?id=ml5bx1PxxOQC.
- ^ Oparil, Suzanne; Weber, Michael (22 April 2005). Hypertension: a companion to Brenner and Rector's the kidney (2 ed.). Philadelphia: Elsevier Mosby. p. 816. ISBN 978-0-7216-0258-5. http://books.google.com/?id=6YUJXURDEZkC.
- ^ Riviere, Jim; Papich, Mark G. (30 Mar 2009). Veterinary Pharmacology and Therapeutics. Wiley-Blackwell. p. 358. ISBN 978-0-8138-2061-3. http://books.google.com/?id=ievLulSqwBAC.
- ^ International Narcotics Control Board (August 2003). "List of psychotropic substances under international control" (PDF). incb.org. http://www.incb.org/pdf/e/list/green.pdf. Retrieved 2008-12-17.
- ^ Blackpool NHS Primary Care Trust (2007). "Medicines Management Update" (PDF). United Kingdom: National Health Service. http://www.blackpool.nhs.uk/images/uploads/CD-update-GP-v2-may08.pdf.
External links
Anticonvulsants (N03) GABAA receptor agonist Clobazam • Clonazepam • Clorazepate • Diazepam# • Flutoprazepam • Lorazepam • Midazolam • Nimetazepam • Nitrazepam • TemazepamOther GABA agents Carbonic anhydrase inhibitor Channel blockers Primarily sodiumPrimarily calciumUnknown/ungroupedChannel openers PotassiumRetigabineIndirect GABA agents GABA transaminase inhibitor: Valproic acid# (Sodium valproate & Valproate semisodium) • Valpromide • Valnoctamide • Valproate pivoxil
GABA reuptake inhibitor: TiagabineUnknown/multiple/
unsortedPropionates#WHO-EM. ‡Withdrawn from market. Clinical trials: †Phase III. §Never to phase III
Anesthesia Types Techniques Measurements Instruments Anaesthetic machine · Boyle's machine · Gas cylinder · IoC-View monitor · Laryngeal mask airway · Medical monitor · Odom's indicator · Relative analgesia machine · VaporiserDrugs General anaesthetic · Benzodiazepine · Etomidate · FlyNap · Infiltration analgesia · Ketamine · Local anesthetic · Methohexital · Midazolam · Neuraxial blockade · Propofol · Thiopental · ThiopentoneComplications Allergic reactions · Anesthesia awareness · Local anesthetic toxicity · Perioperative mortality · Malignant hyperthermiaFields of study Professions Anaesthetic technician · Anesthesiologist · Certified Anesthesia Technician · Certified Anesthesia Technologist · Nurse anaesthetistHistory A.C.E. mixture · Helsinki Declaration for Patient Safety in Anaesthesiology · History of tracheal intubationOrganizations American Society of Anesthesia Technologists & Technicians · American Society of Anesthesiologists · Anaesthesia Trauma and Critical Care · Association of Anaesthetists of Great Britain and Ireland · Association of Veterinary Anaesthetists · Australian and New Zealand College of Anaesthetists · Australian Society of Anaesthetists · International Anesthesia Research SocietyHypnotics/Sedatives (N05C) GABAA Agonists/PAMs Barbiturates: Allobarbital • Amobarbital • Aprobarbital • Barbital • Butabarbital • Butobarbital • Cyclobarbital • Ethallobarbital • Heptabarbital • Hexobarbital • Mephobarbital • Methohexital • Pentobarbital • Phenobarbital • Proxibarbal • Reposal • Secobarbital • Talbutal • Thiamylal • Thiopental • Vinbarbital • Vinylbital; Benzodiazepines: Brotizolam • Cinolazepam • Climazolam • Doxefazepam • Estazolam • Flunitrazepam • Flurazepam • Flutoprazepam • Haloxazolam • Loprazolam • Lormetazepam • Midazolam • Nimetazepam • Nitrazepam • Quazepam • Temazepam • Triazolam; Carbamates: Carisoprodol • Ethinamate • Hexapropymate • Meprobamate • Methocarbamol • Procymate • Tybamate; Neuroactive Steroids: Acebrochol • Allopregnanolone • Alphadolone • Alphaxolone • Eltanolone • Ganaxolone • Hydroxydione • Minaxolone • Org 20599 • Org 21465 • Tetrahydrodeoxycorticosterone; Nonbenzodiazepines: CL-218,872 • Eszopiclone • Indiplon • JM-1232 • Lirequinil • Necopidem • Pazinaclone • ROD-188 • Saripidem • Suproclone • Suriclone • SX-3228 • U-89843A • U-90042 • Zaleplon • Zolpidem • Zopiclone; Phenols: Fospropofol • Propofol; Piperidinediones: Glutethimide • Methyprylon • Pyrithyldione • Piperidione; Quinazolinones: Afloqualone • Cloroqualone • Diproqualone • Etaqualone • Mebroqualone • Mecloqualone • Methaqualone • Methylmethaqualone • Nitromethaqualone; Others: 2-Methyl-2-butanol • Acetophenone • Acetylglycinamide chloral hydrate • Bromide (Lithium bromide, Potassium bromide, Sodium bromide) • Centalun • Chloral hydrate • Chloralose • Chloralodol • Clomethiazole • Dichloralphenazone • Ethanol (Alcohol) • Ethchlorvynol • Etomidate • Gaboxadol • Loreclezole • Methylpentynol • Metomidate • Paraldehyde • Petrichloral • Sulfonmethane • Trichloroethanol • Triclofos • Valerenic acid (Valerian)GABAB Agonists H1 Inverse agonists Antihistamines: Captodiame • Cyproheptadine • Dimenhydrinate • Diphenhydramine • Doxylamine • Hydroxyzine • Methapyrilene • Pheniramine • Promethazine • Propiomazine; Others: Tricyclic antidepressants (Amitriptyline, Doxepin, Trimipramine, etc.) • Tetracyclic antidepressants (Mianserin, Mirtazapine, etc.) • Typical antipsychotics (Chlorpromazine, Thioridazine, etc.) • Atypical antipsychotics (Olanzapine, Quetiapine, Risperidone, etc.)α1-Adrenergic Antagonists Mianserin • Niaprazine • Trazodone; Others: Tricyclic antidepressants (Amitriptyline, Doxepin, Trimipramine, etc.) • Typical antipsychotics (Chlorpromazine, Thioridazine, etc.) • Atypical antipsychotics (Olanzapine, Quetiapine, Risperidone, etc.)α2-Adrenergic Agonists 4-NEMD • Clonidine • Detomidine • Dexmedetomidine • Lofexidine • Medetomidine • Romifidine • Tizanidine • Xylazine5-HT2A Antagonists Eplivanserin • Niaprazine • Pruvanserin • Trazodone • Volinanserin; Others: Tricyclic antidepressants (Amitriptyline, Doxepin, Trimipramine, etc.) • Tetracyclic antidepressants (Mianserin, Mirtazapine, etc.) • Typical antipsychotics (Chlorpromazine, Thioridazine, etc.) • Atypical antipsychotics (Olanzapine, Quetiapine, Risperidone, etc.)Melatonin Agonists Orexin Antagonists Others Acecarbromal • Apronal • Bromisoval • Cannabidiol (Cannabis) • Carbromal • Embutramide • Evoxine • Fenadiazole • Gabapentin • Kavalactones (Kava) • Mephenoxalone • Opiates/Opioids (Hydrocodone, Morphine (Opium), etc.) • Passion flower • Scopolamine (Mandrake) • ValnoctamideCategories:- Imidazobenzodiazepines
- Organofluorides
- Hoffmann-La Roche
Wikimedia Foundation. 2010.

