- Methyprylon
-
Methyprylon 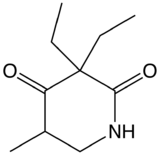
Systematic (IUPAC) name (RS)-3,3-diethyl-5-methylpiperidine-2,4-dione Clinical data Trade names Dimerin, Methyprylone, Noctan, Noludar Pregnancy cat. ? Legal status Schedule III (US) Routes oral Pharmacokinetic data Protein binding 60% Half-life 6-16 hours Identifiers CAS number 125-64-4 
ATC code N05CE02 PubChem CID 4162 DrugBank APRD00734 ChemSpider 4018 
UNII CUT48I42ON 
KEGG D01150 
ChEMBL CHEMBL1200790 
Chemical data Formula C10H17NO2 Mol. mass 183.248 g/mol  (what is this?) (verify)
(what is this?) (verify)Methyprylon (Noludar) is a sedative of the piperidinedione derivative family developed by Hoffmann-La Roche[1]. This medicine was used for treating insomnia, but is now rarely used as it has been replaced by newer drugs with fewer side effects, such as benzodiazepines.[2] Methyprylon was withdrawn from the US market in June 1989 and the Canadian market in September 1990.
Contents
Adverse effects
Side effects can include: Skin rash, fever, depression, ulcers or sores in mouth or throat, unusual bleeding or bruising, confusion, fast heartbeat, respiratory depression, swelling of feet or lower legs, dizziness, drowsiness, headache, double vision, clumsiness, constipation, diarrhea, nausea, vomiting, unusual weakness.[citation needed]
Pharmacokinetics
A study of single oral doses of 300 mg in healthy volunteers found that the zero-order absorption model fit the data best. Mean (+/- SD) values for the half-life (9.2 +/- 2.2 h), apparent clearance, (11.91 +/- 4.42 mL/h/kg) and apparent steady-state volume of distribution, (0.97 +/- 0.33 L/kg) were found.[3]
A case report found the pharmacokinetics of methyprylon nonlinear (concentration dependent) in an overdose case; explanations included saturation or inhibition of metabolic pathways. The generally accepted half-life for a therapeutic dose was not found appropriate in intoxicated patients and would underestimate the time required to reach a safe concentration of the drug.[4]
Synthesis
Methyprylon may be synthesized starting from an aldol condensation of ethyl 2,2-diethyl-3-oxobutanoate with ethyl formate. The resulting enol is converted to the lactam pyrithyldione by treating with ammonia and heating. The lactam is formylated at postion-5 and reduced to yield methyprylon.[5]

See also
References
- ^ US patent 2680116, Frick, H. & Lutz, A. H., "Piperidiones and Process for the Manufacture thereof", issued 1954-06-01, assigned to Hoffmann-La Roche
- ^ Lomen, P.; Linet, O. I. (1976). "Hypnotic efficacy of triazolam and methyprylon in insomniac in-patients". The Journal of international medical research 4 (1): 55–58. PMID 16792.
- ^ Gwilt, P. R.; Pankaskie, M. C.; Thornburg, J. E.; Zustiak, R.; Shoenthal, D. R. (1985). "Pharmacokinetics of methyprylon following a single oral dose". Journal of pharmaceutical sciences 74 (9): 1001–1003. PMID 2866242.
- ^ Contos, D. A.; Dixon, K. F.; Guthrie, R. M.; Gerber, N.; Mays, D. C. (1991). "Nonlinear elimination of methyprylon (noludar) in an overdosed patient: Correlation of clinical effects with plasma concentration". Journal of pharmaceutical sciences 80 (8): 768–771. PMID 1686463.
- ^ Schnider, O.; Frick, H.; Lutz, A. H. (1954). "Synthesis of new hypnotics from pyridine and piperidine groups". Experientia 10 (3): 135–137. PMID 13161893.
Categories:- Medicine stubs
- Piperidines
- Lactams
- Ketones
Wikimedia Foundation. 2010.
