- Lactam
-
A lactam (the noun is a portmanteau of the words lactone + amide) is a cyclic amide. Prefixes indicate how many carbon atoms (apart from the carbonyl moiety) are present in the ring: β-lactam (2 carbon atoms outside the carbonyl, 4 ring atoms in total), γ-lactam (3 and 5), δ-lactam (4 and 6). Beta β, gamma γ and delta δ are the second, third and fourth letters in the alphabetical order of the Greek alphabet, respectively.
Contents
Synthesis
General synthetic methods exist for the organic synthesis of lactams.
- Lactams form by the acid-catalyzed rearrangement of oximes in the Beckmann rearrangement.
- Lactams form from cyclic ketones and hydrazoic acid in the Schmidt reaction.
- Lactams form from cyclisation of amino acids.
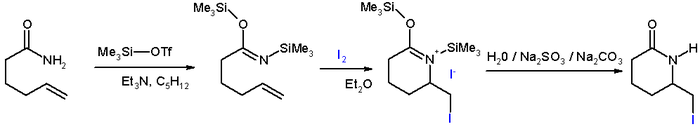
- In iodolactamization [1] an iminium ion reacts with an halonium ion formed in situ by reaction of an alkene with iodine.
- Lactams form by copper catalyzed 1,3-dipolar cycloaddition of alkynes and nitrones in the Kinugasa reaction
- Diels-Alder reaction between cyclopentadiene and chlorosulfonyl isocyanate (CSI) can be utilized to obtain both β- as well as γ-lactam. At lower temp (-78 oC) β-lactam is the preferred product. At optimum temperatures, a highly useful γ-lactam known as Vince Lactam is obtained.[2]
Tautomerization to Lactim
Lactim is a cyclic carboximidic acid compound characterized by an endocyclic carbon-nitrogen double bond. It is formed when lactam undergoes tautomerization.
Reactions
- Lactams can polymerize to polyamides.
See also
- β-lactam with a four-membered ring found in beta-lactam antibiotics. Penicillin, considered the most famous antibiotic, is a β-lactam antibiotic.
- Lactone, a cyclic ester.
- Caprolactam
References
- ^ Spencer Knapp, Frank S. Gibson Organic Syntheses, Coll. Vol. 9, p.516 (1998); Vol. 70, p.101 (1992) Online article
- ^ Pham, P.-T.; Vince, R. Phosphorus, Sulphur and Silicon 2007, 779-791.
Categories:- Functional groups
- Lactams
Wikimedia Foundation. 2010.