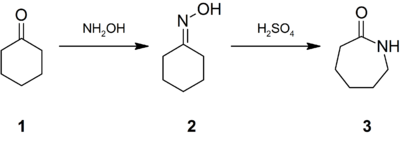- Caprolactam
-
Caprolactam 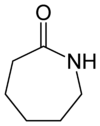
 Azepan-2-oneOther namesε-Caprolactam, 1-Aza-2-cycloheptanone, 2-Azacycloheptanone, Capron PK4, Cyclohexanone iso-oxime, Extrom 6N, Hexahydro-2-azepinone, Hexahydro-2H-azepin-2-one (9CI), Hexanolactame
Azepan-2-oneOther namesε-Caprolactam, 1-Aza-2-cycloheptanone, 2-Azacycloheptanone, Capron PK4, Cyclohexanone iso-oxime, Extrom 6N, Hexahydro-2-azepinone, Hexahydro-2H-azepin-2-one (9CI), HexanolactameIdentifiers CAS number 105-60-2 
PubChem 7768 ChemSpider 7480 
UNII 6879X594Z8 
EC number 203-313-2 KEGG C06593 
ChEBI CHEBI:28579 
ChEMBL CHEMBL276218 
Jmol-3D images Image 1 - O=C1NCCCCC1
Properties Molecular formula C6H11NO Molar mass 113.16 g/mol Appearance white solid Density 1,01 g/cm3 Melting point 68 °C
Boiling point 136-138 °C @ 10 mm Hg
Solubility in water 820 g/L (20 °C) Hazards R-phrases R20, R22, R36/37/38 Flash point 125 °C  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Caprolactam is an organic compound with the formula (CH2)5C(O)NH. This colourless solid is a lactam or a cyclic amide of caproic acid. Approximately 2 billion kilograms are produced annually. Caprolactam is the precursor to Nylon 6, a widely used synthetic polymer.[1]
Contents
Synthesis and production
Caprolactam was first described in the late 1800s when it was prepared by the cyclization of ε-aminocaproic acid, the product of the hydrolysis of caprolactam. Given the commercial significance of nylon-6, many methods have been developed for the production of caprolactam. Most of the caprolactam is synthesised from cyclohexanone (1), which is first converted to its oxime (2). Treatment of this oxime with acid induces the Beckmann rearrangement to give caprolactam (3):
The immediate product of the acid-induced rearrangement is the bisulfate salt of caprolactam. This salt is neutralized with ammonia to release the free lactam and cogenerate ammonium sulfate. In optimizing the industrial practices, much attention is directed toward minimizing the production of ammonium salts.
The other major industrial route involves formation of the oxime from cyclohexane using nitrosyl chloride. The advantage of this method is that cyclohexane is less expensive than cyclohexanone. In earlier times, caprolactam was prepared by treatment of caprolactone with ammonia.[1]
Uses
Caprolactam is the precursor to Nylon-6. The conversion entails a ring-opening polymerization:
- n (CH2)5C(O)NH → [(CH2)5C(O)NH]n
Nylon-6 is widely used in fibers and plastics.
Safety
Caprolactam is an irritant and is mildly toxic, with an LD50 of 1.1 g/kg (rat, oral). In 1991, it was included on the list of hazardous air pollutants by the U.S. Clean Air Act of 1990. It was subsequently removed from the list in 1993.[2] In water, caprolactam hydrolyzes to aminocaproic acid, which is used medicinally.
As of 2011 caprolactam had the unusual status of being the only chemical in the International Agency for Research on Cancer's lowest hazard category, Group 4 "probably not carcinogenic to humans".[3][4]
References
- ^ a b Josef Ritz, Hugo Fuchs, Heinz Kieczka, William C. Moran "Caprolactam" Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2002. doi:10.1002/14356007.a05_031
- ^ EPA - Modifications To The 112(b)1 Hazardous Air Pollutants
- ^ World Health Organisation verdict on mobile phones and cancer, Cancer Research UK, May 31 2011, http://scienceblog.cancerresearchuk.org/2011/05/31/who-verdict-on-mobile-phones-and-cancer/, retrieved 2011-06-01
- ^ Agents Classified by the IARC Monographs, Volumes 1–102, WHO, http://monographs.iarc.fr/ENG/Classification/ClassificationsGroupOrder.pdf, retrieved July 15, 2011
Carcinogen—Cancer causing materials and agents Main articles Major suspected carcinogens IARC International Agency for Research on Cancer / IARC agent lists: Group 1 · Group 2A · Group 2B · Group 3 · Group 4 (Caprolactam)Categories:- Azepanes
- Lactams
- Monomers
Wikimedia Foundation. 2010.