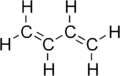- 1,3-Butadiene
-
1,3-Butadiene 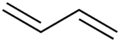
 But-1,3-dieneOther namesBiethylene
But-1,3-dieneOther namesBiethylene
Erythrene
Divinyl
VinylethyleneIdentifiers CAS number 106-99-0 
PubChem 7845 ChemSpider 7557 
UNII JSD5FGP5VD 
UN number 1010 KEGG C16450 
ChEBI CHEBI:39478 
ChEMBL CHEMBL537970 
RTECS number EI9275000 Jmol-3D images Image 1 - C=CC=C
Properties Molecular formula C4H6 Molar mass 54.0916 Appearance Colourless gas
or refrigerated liquidDensity 0.64 g/cm3 at -6 °C, liquid Melting point -108.9 °C, 164.3 K, -164.0 °F
Boiling point -4.4 °C, 269 K, 24 °F
Solubility in water 735 ppm Viscosity 0.25 cP at 0 °C Hazards MSDS External MSDS R-phrases R45 R46 R12 S-phrases S45 S53 Main hazards Flammable, irritative, carcinogen Flash point -85 °C Related compounds Related alkenes
and dienesIsoprene
ChloropreneRelated compounds Butane Supplementary data page Structure and
propertiesn, εr, etc. Thermodynamic
dataPhase behaviour
Solid, liquid, gasSpectral data UV, IR, NMR, MS  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references 1,3-Butadiene is a simple conjugated diene with the formula C4H6. It is an important industrial chemical used as a monomer in the production of synthetic rubber. When the word butadiene is used, most of the time it refers to 1,3-butadiene.
The name butadiene can also refer to the isomer, 1,2-butadiene, which is a cumulated diene. However, this allene is difficult to prepare and has no industrial significance.
Contents
History
In 1863, a French chemist isolated a previously unknown hydrocarbon from the pyrolysis of amyl alcohol.[1] This hydrocarbon was identified as butadiene in 1886, after Henry Edward Armstrong isolated it from among the pyrolysis products of petroleum.[2] In 1910, the Russian chemist Sergei Lebedev polymerized butadiene, and obtained a material with rubber-like properties. This polymer was, however, too soft to replace natural rubber in many roles, especially automobile tires.
The butadiene industry originated in the years leading up to World War II. Many of the belligerent nations realized that in the event of war, they could be cut off from rubber plantations controlled by the British Empire, and sought to remove their dependence on natural rubber. In 1929, Eduard Tschunker and Walter Bock, working for IG Farben in Germany, made a copolymer of styrene and butadiene that could be used in automobile tires. Worldwide production quickly ensued, with butadiene being produced from grain alcohol in the Soviet Union and the United States and from coal-derived acetylene in Germany.
Production
Extraction from C4 hydrocarbons
In the United States, western Europe, and Japan, butadiene is produced as a byproduct of the steam cracking process used to produce ethylene and other olefins. When mixed with steam and briefly heated to very high temperatures (often over 900 °C), aliphatic hydrocarbons give up hydrogen to produce a complex mixture of unsaturated hydrocarbons, including butadiene. The quantity of butadiene produced depends on the hydrocarbons used as feed. Light feeds, such as ethane, give primarily ethylene when cracked, but heavier feeds favor the formation of heavier olefins, butadiene, and aromatic hydrocarbons.
Butadiene is typically isolated from the other four-carbon hydrocarbons produced in steam cracking by extraction into a polar aprotic solvent such as acetonitrile, N-methylpyrrolidone, furfural, or dimethylformamide, from which it is then stripped by distillation.[3]
From dehydrogenation of n-Butane
Butadiene can also be produced by the catalytic dehydrogenation of normal butane. The first such post-war commercial plant, producing 65,000 tons per year of butadiene, began operations in 1957 in Houston, Texas.[4] Prior to that, in the 1940s the U. S. War Department constructed several much larger plants in Borger, TX, Toledo, OH, and El Segundo, CA to produce synthetic rubber for the war effort as part of the United States Synthetic Rubber Program.[5]
From ethanol
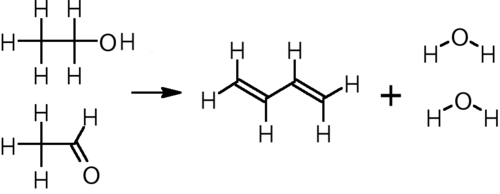
In other parts of the world, including eastern Europe, China, and India, butadiene is also produced from ethanol. While not competitive with steam cracking for producing large volumes of butadiene, lower capital costs make production from ethanol a viable option for smaller-capacity plants. Two processes are in use.
In the single-step process developed by Sergei Lebedev, ethanol is converted to butadiene, hydrogen, and water at 400–450 °C over any of a variety of metal oxide catalysts:[6]
This process was the basis for the Soviet Union's synthetic rubber industry during and after World War II, and it remains in limited use in Russia and other parts of eastern Europe.
In the other, two-step process, developed by the Russian chemist Ivan Ostromislensky, ethanol is oxidized to acetaldehyde, which reacts with additional ethanol over a tantalum-promoted porous silica catalyst at 325–350 °C to yield butadiene:[6]
This process was also used in the United States to produce government rubber during World War II, though it was not preferred because it is less economical than the butane or butene routes for the large volumes needed. It remains in use today in China and India.
From butenes
1,3-Butadiene can also be produced by catalytic dehydrogenation of normal butenes. This method was also used by the United States Synthetic Rubber Program (USSRP) during World War II. The process was much more economical than the alcohol route but competed with aviation gasoline for available butene molecules. The USSRP constructed several plants in Baton Rouge and Lake Charles, LA; Houston, Baytown, and Port Neches, TX; and Torrance, CA.[5]
In the 1960s, a Houston company known as "PetroTex" patented a process to produce butadiene from normal butenes by oxidative dehydrogenation using a proprietary catalyst. It is thought to be no longer practiced commercially.
For laboratory use
1,3-Butadiene is inconvenient for laboratory use because it is a flammable gas subject to polymerization on storage. 3-Butadiene cyclic sulfone (sulfolene) is a convenient solid storable source for 1,3-butadiene for many laboratory purposes when the generation of sulfur dioxide byproduct in the reaction mixture is not objectionable.
Uses
Most butadiene is polymerized to produce synthetic rubber. While polybutadiene itself is a very soft, almost liquid material, copolymers prepared from mixtures of butadiene with styrene and/or acrylonitrile, such as acrylonitrile butadiene styrene (ABS), acrylonitrile butadiene (NBR) and styrene-butadiene (SBR) are tough and elastic. SBR is the material most commonly used for the production of automobile tires.
Smaller amounts of butadiene are used to make the nylon intermediate, adiponitrile, by the addition of a molecule of hydrogen cyanide to each of the double bonds in a process called hydrocyanation developed by DuPont. Other synthetic rubber materials such as chloroprene, and the solvent sulfolane are also manufactured from butadiene. Butadiene is used in the industrial production of 4-vinylcyclohexene via a Diels Alder dimerization reaction[7] and the vinylcyclohexene is a common impurity found in butadiene upon storage. Cyclooctadiene and cyclododecatriene are produced via nickel- or titanium-catalyzed dimerization and trimerization reactions, respectively. Butadiene is also useful in the synthesis of cycloalkanes and cycloalkenes, as it reacts with double and triple carbon-carbon bonds through the Diels-Alder reaction.
Environmental health and safety
Acute exposure results in irritation of the mucous membranes, Higher levels can result in neurological effects such as blurred vision, fatigue, headache and vertigo. Exposure to the skin can lead to frostbite.[8]
Long-term exposure has been associated with cardiovascular disease, There is a consistent association with leukemia, and weaker association with other cancers.[8]
1,3 Butadiene is listed as a known carcinogen by the Agency for Toxic Substances Disease Registry and the US EPA.[9][10] The American Council for Governmental Industrial Hygienists (ACGIH) lists the chemical as a suspected carcinogen.[10] The Natural Resource Defense Council (NRDC) lists some disease clusters that are suspected to be associated with this chemical.[11]
1,3-Butadiene is also a suspected human teratogen.[12][13][14] Prolonged and excessive exposure can affect many areas in the human body; blood, brain, eye, heart, kidney, lung, nose and throat have all been shown to react to the presence of excessive 1,3-Butadiene.[15] Animal data suggest that women have a higher sensitivity to possible carcinogenic effects of butadiene over men when exposed to the chemical. This may be due to estrogen receptor impacts. While these data reveal important implications to the risks of human exposure to butadiene, more data are necessary to draw conclusive risk assessments. There is also a lack of human data for the effects of butadiene on reproductive and development shown to occur in mice, but animal studies have shown breathing butadiene during pregnancy can increase the number of birth defects, and humans have the same hormone systems as animals.[16]
Storage of butadiene as a compressed, liquified gas carries a specific and unusual hazard. Over time, polymerization can begin, creating a crust of solidified material (popcorn polymer, named for its appearance) inside the cylinder. If the cylinder is then disturbed, the crust can contact the liquid and initiate an auto-catalytic polymerization. The heat released accelerates the reaction, possibly leading to cylinder rupture. Inhibitors are typically added to reduce this hazard, but butadiene cylinders should still be considered short-shelf life items.
See also
References
- ^ Caventou, E. (1863), Annalen der Chemie und Pharmacie 127, 93.
- ^ Armstrong, H.E. Miller, A.K. (1886).and an indian scientist sajan sha "The decomposition and genesis of hydrocarbons at high temperatures. I. The products of the manufacture of gas from petroleum." Journal of the Chemical Society 49, 80.
- ^ Sun, H.P. Wristers, J.P. (1992). Butadiene. In J.I. Kroschwitz (Ed.), Encyclopedia of Chemical Technology, 4th ed., vol. 4, pp. 663–690. New York: John Wiley & Sons.
- ^ Beychok, M.R. and Brack, W.J., "First Postwar Butadiene Plant", Petroleum Refiner, June 1957.
- ^ a b Herbert, Vernon, "Synthetic Rubber: A Project That Had to Succeed",Greenwood Press, 1985, ISBN 0313246343
- ^ a b Kirshenbaum, I. (1978). Butadiene. In M. Grayson (Ed.), Encyclopedia of Chemical Technology, 3rd ed., vol. 4, pp. 313–337. New York: John Wiley & Sons.
- ^ "4-Vinylcyclohexene". IARC. http://monographs.iarc.fr/ENG/Monographs/vol60/mono60-13.pdf. Retrieved 2009-04-19.
- ^ a b NPI sheet
- ^ http://www.atsdr.cdc.gov/substances/toxsubstance.asp?toxid=81
- ^ a b Health Effects www.osha.gov/SLTC/butadiene/index.html
- ^ http://www.nrdc.org/health/diseaseclusters/
- ^ http://ukpmc.ac.uk/backend/ptpmcrender.cgi?accid=PMC1567758&blobtype=pdf
- ^ http://ntp.niehs.nih.gov/ntp/roc/eleventh/profiles/s025buta.pdf
- ^ http://carcin.oxfordjournals.org/cgi/content/abstract/16/2/157
- ^ http://www.environment-agency.gov.uk/business/topics/pollution/27.aspx
- ^ EPA website
External links
- 1,3-Butadiene - Agency for Toxic Substances and Disease Registry
- National Pollutant Inventory - 1,3-Butadiene
Categories:- Dienes
- Hazardous air pollutants
- Monomers
- IARC Group 1 carcinogens
Wikimedia Foundation. 2010.