- Cyclobutadiene
-
Cyclobutadiene 
 1,3-CyclobutadieneOther namesCyclobutadiene
1,3-CyclobutadieneOther namesCyclobutadiene
[4]AnnuleneIdentifiers CAS number 1120-53-2 PubChem 136879 ChemSpider 120626 
ChEBI CHEBI:33657 
Jmol-3D images Image 1
Image 2- C1=CC=C1
C1=CC=C1
Properties Molecular formula C4H4 Molar mass 52.07 g/mol  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Cyclobutadiene is the smallest [n]-annulene ([4]-annulene), an extremely unstable hydrocarbon having a lifetime shorter than five seconds in the free state. It has chemical formula C4H4 and a rectangular structure verified by infrared studies. This is in contrast to the square geometry predicted by simple Hückel theory[citation needed]. Though it has alternating single and double bonds, it fails Hückel's rule, because its ring has 4 π-electrons, and 4 is not twice an odd number. Some cyclobutadiene-metal compounds are stable because the metal atom provides 2 more electrons to the system.
The pi electron energy of cyclobutadiene is higher than that of its open-chain counterpart, 1,3-butadiene, and it is therefore said to be anti-aromatic rather than aromatic. Recent studies on cyclobutadiene show that it has a rectangular structure as opposed to a square structure and two different 1,2-dideuterio-1,3-cyclobutadiene stereoisomers. This indicates that the pi electrons are localized and therefore not considered to be antiaromatic. However it is far from stable, it is highly reactive and has a very short lifetime. Cyclobutadiene dimerizes by a Diels-Alder reaction at 35 K. The monomeric form has been studied at higher temperatures by trapping with matrix isolation in a noble gas.
Synthesis
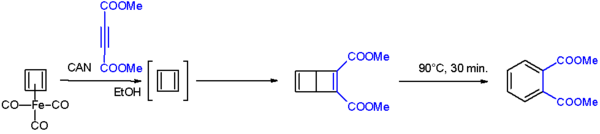
After numerous attempts, it was first synthesized in 1965 by Rowland Pettit of the University of Texas, although he could not isolate it. Cyclobutadiene can be generated through degradation from a cyclobutadiene metal compound for example C4H4Fe(CO)3 with ammonium cerium(IV) nitrate. This cyclobutadieneiron tricarbonyl complex was prepared from Fe4(CO)9 and cis-dichlorocyclobutene in a double dehydrohalogenation.[1][2]
Cyclobutadiene when liberated from the iron complex reacts with electron-deficient alkynes to a Dewar benzene:[3]
The Dewar benzene converts to dimethyl phthalate on heating at 90°C.
One cyclobutadiene derivative is also accessible through a [2+2]cycloaddition of a di-alkyne. In this particular reaction the trapping reagent is 2,3,4,5-tetraphenylcyclopenta-2,4-dienone and one of the final products (after expulsion of carbon monoxide) is a cyclooctatetraene:[4]
See also
- Cyclobutene
- Butadiene
References
- ^ Cyclobutadiene- and Benzocyclobutadiene-Iron Tricarbonyl Complexes G. F. Emerson, L. Watts, R. Pettit; J. Am. Chem. Soc.; 1965; 87(1); 131-133. First Page
- ^ Iron, tricarbonyl (η4-1,3-cyclobutadiene)- R. Pettit and J. Henery Organic Syntheses, Coll. Vol. 6, p.310 (1988); Vol. 50, p.21 (1970) Link
- ^ Cyclobutadiene L. Watts, J. D. Fitzpatrick, R. Pettit J. Am. Chem. Soc.; 1965; 87(14); 3253-3254. Abstract
- ^ Revisit of the Dessy-White Intramolecular Acetylene-Acetylene [2 + 2] Cycloadditions Chung-Chieh Lee, Man-kit Leung, Gene-Hsiang Lee, Yi-Hung Liu, and Shie-Ming Peng J. Org. Chem.; 2006; 71(22) pp 8417 - 8423; (Article) doi:10.1021/jo061334v
Alkenes Dienes Cyclobutadiene · Cyclopentadiene · Cyclohexadiene (1,3-Cyclohexadiene · 1,4-Cyclohexadiene) · Cycloheptadiene · Cyclooctadiene (1,5-Cyclooctadiene)Categories:- Annulenes
- Antiaromatic compounds
- C1=CC=C1
Wikimedia Foundation. 2010.

![Acetylene-Acetylene [2 + 2] Cycloadditions Chung-Chieh Lee 2006](/pictures/enwiki/52/400px-CyclobutadienSynthDessyWhite.png)