- Cyclooctatetraene
-
Cyclooctatetraene 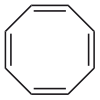
 1,3,5,7-cyclooctatetraeneOther namesCOT, [8]-annulene
1,3,5,7-cyclooctatetraeneOther namesCOT, [8]-annuleneIdentifiers CAS number 629-20-9 
ChemSpider 553448 
RTECS number CY1400000 Jmol-3D images Image 1 - C1=C\C=C/C=C\C=C1
Properties Molecular formula C8H8 Molar mass 104.15 g/mol Appearance Clear yellow Density 0.9250 g/cm3, liquid Melting point -5 – -3 °C (268 – 270 K)
Boiling point 142 – 143 °C (415 – 416 K)
Solubility in water immiscible Hazards EU classification Flammable (F)
Carc. Cat. 1
Muta. Cat. 2
Toxic (T)R-phrases R45, R46, R11, R36/38,
R48/23/24/25, R65S-phrases S53, S45 NFPA 704 Flash point -11 °C Autoignition
temperature561 °C Related compounds Related hydrocarbons Cyclooctane
Tetraphenylene (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references 1,3,5,7-Cyclooctatetraene (COT) is an unsaturated derivative of cyclooctane, with the formula C8H8. It is also known as [8]annulene. This polyunsaturated hydrocarbon is a colorless to light yellow flammable liquid at room temperature. Because of its stoichiometric relationship to benzene, COT has been the subject of much research and some controversy.
Unlike benzene, C6H6, however, cyclooctatetraene, C8H8, is not aromatic, although its dianion, C8H82- (cyclooctatetraenide), is. Its reactivity is characteristic of an ordinary polyene, i.e. it undergoes addition reactions. Benzene, by contrast, characteristically undergoes substitution reactions, much as alkanes do, not additions.
Contents
History
1,3,5,7-Cyclooctatetraene was initially synthesized by Richard Willstätter at Munich in 1905:[1][2]
Willstätter noted that the compound did not exhibit the expected aromaticity. Between 1939 and 1943, chemists throughout the US unsuccessfully attempted to synthesize COT. They rationalized their lack of success with the conclusion that Willstätter had not actually synthesized the compound but instead its isomer, styrene. Willstätter responded to these reviews in his autobiography, where he noted that the American chemists were 'untroubled' by the reduction of his cyclooctatetraene to cyclooctane (a reaction impossible for styrene). In 1947, Walter Reppe at Ludwigshafen at last repeated Willstätter's synthesis.[3]
Structure and bonding
Early studies demonstrated that COT did not display the chemistry of an aromatic compound.[4] Then, early electron diffraction experiments concluded that the C-C bond distances were identical.[5] However, X-ray diffraction data from H. S. Kaufman demonstrated cyclooctatetraene to adopt several conformations and to contain two distinct C-C bond distances.[6] This result indicated that COT is an annulene with fixed alternating single and double C-C bonds.
In its normal state, cyclooctatetraene is non-planar and adopts a tub conformation with angles C=C-C = 126.1° and C=C-H = 117.6° (of the Cx atom in C-HCx=C bound).[7]
Synthesis
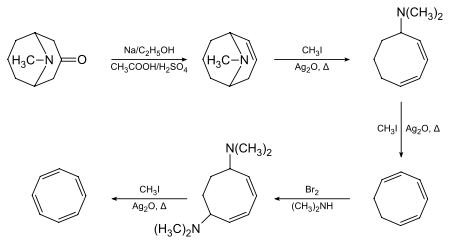
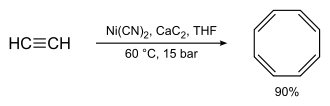
Richard Willstätter's original synthesis (4 consecutive elimination reactions on a cyclooctane framework) gives relatively low yields. Reppe's synthesis of cyclooctatetraene, which involves treating acetylene at high pressure with a warm mixture of nickel cyanide and calcium carbide, was much better, with chemical yields near 90%:[3]
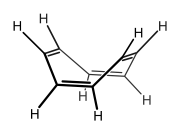
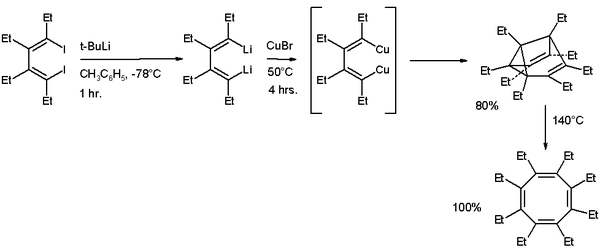
COT can also be prepared by photolysis of barrelene, one its structural isomers, the reaction proceeding via another isolable isomer, semibullvalene.[8] COT derivatives can also be synthesised by way of semibullvalene intermediates. In the sequence illustrated below, octaethylcyclooctatetraene (C8Et8) is formed by thermal isomerisation of octaethylsemibullvalene, itself formed by copper(I) bromide mediated cyclodimerisation of 1,2,3,4-tetraethyl-1,4-dilithio-1,3-butadiene.[9]
Because COT is unstable and easily forms explosive organic peroxides, a small amount of hydroquinone is usually added to commercially available material. Testing for peroxides is advised when using a previously opened bottle; white crystals around the neck of the bottle may be composed of the peroxide, which may explode when mechanically disturbed.
Natural occurrence
Cyclooctatetraene has been isolated from certain fungi.[10]
Reactions
The π bonds in COT react as usual for olefins, rather than as aromatic ring systems. Mono- and polyepoxides can be generated by reaction of COT with peroxy acids or with dimethyldioxirane. Various other addition reactions are also known. Furthermore, polyacetylene can been synthesized via the ring-opening polymerization of cyclooctatetraene.[11] COT itself —and also analogs with side-chains— have been used as metal ligands and in sandwich compounds.
Cyclooctatetraenide as a ligand
COT readily reacts with potassium metal to form the salt K2COT, which contains the dianion C8H82−.[12] The dianion is both planar in shape and aromatic with a Huckel electron count of 10.
Cyclooctatetraene forms organometallic complexes with some metals, including yttrium and lanthanides. One-dimensional Eu-COT sandwiches have been described as nanowires.[13] The sandwich compounds U(COT)2, or uranocene and Fe(COT)2, are known.
The compound Fe(COT)2, when refluxed in toluene with dimethyl sulfoxide and dimethoxyethane for 5 days, is found to form magnetite and crystalline carbon also containing carbon nanotubes.[14]
Because COT changes conformation between tub-shaped and planar with addition or subtraction of electrons, it could, in principle, be used to construct artificial muscles. Such devices have been contemplated to be makeable by grafting COT derivatives to a backbone of a suitable conducting polymer, which would supply or remove the reducing equivalents.[15]
See also
- Cyclobutadiene
- Semibullvalene and barrelene, structural isomers of cyclooctatetraene
- Benzene
References
- ^ Mason, S. "The Science and Humanism of Linus Pauling (1901-1994)", Chemical Society Reviews 26, 1 (February 1997).
- ^ Richard Willstätter, Ernst Waser (1911). "Über Cyclo-octatetraen". Berichte der deutschen chemischen Gesellschaft 44 (3): 3423–3445. doi:10.1002/cber.191104403216.
- ^ a b Walter Reppe, Otto Schlichting, Karl Klager, Tim Toepel (1948). "Cyclisierende Polymerisation von Acetylen I Über Cyclooctatetraen". Justus Liebigs Annalen der Chemie 560 (1): 1–92. doi:10.1002/jlac.19485600102.
- ^ Johnson, A.W., Sci. Progress; 506; 1947; 35.
- ^ Bastiensen, O.; Hassel, O.; Langseth, A. (1947). "The ‘Octa-Benzene’, Cyclo-octatetraene (C8H8)". Nature 160 (4056): 128. doi:10.1038/160128a0. http://www.nature.com/nature/journal/v160/n4056/pdf/160128a0.pdf.
- ^ Kaufman, H. S.; Fankuchen, I.; Mark, H. (1948). "Structure of Cyclo-octatetraene". Nature 161 (4083): 165. doi:10.1038/161165a0. http://www.nature.com/nature/journal/v161/n4083/pdf/161165a0.pdf.
- ^ Thomas, P. M.; Weber, A. (1978). "High resolution Raman spectroscopy of gases with laser sources. XIII - the pure rotational spectra of 1,3,5,7-cyclooctatetraene and 1,5-cyclooctadiene". Journal of Raman Spectroscopy 7 (6): 353–357. doi:10.1002/jrs.1250070614.
- ^ Zimmerman, H. E.; Grunewald, G. L. (1966). "The Chemistry of Barrelene. III. A Unique Photoisomerization to Semibullvalene". J. Am. Chem. Soc. 88 (1): 183–184. doi:10.1021/ja00953a045. http://pubs.acs.org/cgi-bin/abstract.cgi/jacsat/1966/88/i01/f-pdf/f_ja00953a045.pdf.
- ^ Wang, C.; Yuan, J.; Li, G.; Wang, Z.; Zhang, S.; Xi, Z. (2006). "Metal-Mediated Efficient Synthesis, Structural Characterization, and Skeletal Rearrangement of Octasubstituted Semibullvalenes". J. Am. Chem. Soc. 128 (14): 4564–4565. doi:10.1021/ja0579208. PMID 16594680.
- ^ Stinson, M.; Ezra, D.; Hess, W. M.; Sears, J.; Strobel, G. “An endophytic Gliocladium sp. of Eucryphia cordifolia producing selective volatile antimicrobial compounds” Plant Science 2003, volume 165, pp. 913-922. doi:10.1016/S0168-9452(03)00299-1
- ^ Two Undergraduate Experiments in Organic Polymers: The Preparation of Polyacetylene and Telechelic Polyacetylene via Ring-Opening Metathesis Polymerization Eric J. Moorhead and Anna G. Wenzel Journal of Chemical Education • Vol. 86 No. 8 August 2009 973
- ^ The cyclooctatetraenyl dianion Thomas J. Katz J. Am. Chem. Soc.; 1960; 82(14); 3784-3785. doi:10.1021/ja01499a077
- ^ JST Nanostructed Materials Project Highlights- Prof. Nakajima's Presentation
- ^ Crystalline Graphite from an Organometallic Solution-Phase Reaction Erich C. Walter, Tobias Beetz, Matthew Y. Sfeir, Louis E. Brus, and Michael L. Steigerwald J. Am. Chem. Soc.; 2006; 128(49) pp 15590 - 15591; (Communication) doi: 10.1021/ja0666203
- ^ UCR Fiat Lux: Muscle building - UCR researchers hope to create artificial muscles
Categories:- Annulenes
- Molecular electronics
Wikimedia Foundation. 2010.