- Structural isomer
-
Structural isomerism, or constitutional isomerism (per IUPAC), is a form of isomerism in which molecules with the same molecular formula have bonded together in different orders, as opposed to stereoisomerism.[1] There are multiple synonyms for constitutional isomers.
Three categories of constitutional isomers are skeletal, positional, and functional isomers. Positional isomers are also called regioisomers. Tautomers are subcategory of functional isomers.
Contents
Skeletal isomerism
In skeletal isomerism, or chain isomerism, components of the (usually carbon) skeleton are distinctly re-ordered to create different structures. Pentane exists as three isomers: n-pentane (often called simply "pentane"), isopentane (methylbutane) and neopentane (dimethylpropane).
Skeletal isomerism of pentane 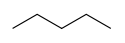
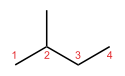
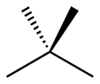
n-Pentane Isopentane Neopentane Position isomerism
In position isomerism a functional group or other substituent changes position on a parent structure. In the table below, the hydroxyl group can occupy three different positions on an n-pentane chain forming three different compounds.
Example of position isomerism 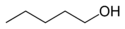
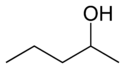
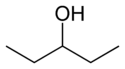
1-Pentanol 2-Pentanol 3-Pentanol Many aromatic isomers exist because substituents can be positioned on different parts of the benzene ring. Only one isomer of phenol or hydroxybenzene exists but cresol or methylphenol has three isomers where the additional methyl group can be placed on three different positions on the ring. Xylenol has one hydroxyl group and two methyl groups and a total of 6 isomers exist.
Positional isomers of xylenol 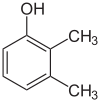
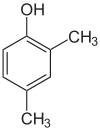
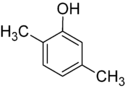
2,3-Xylenol 2,4-Xylenol 2,5-Xylenol 
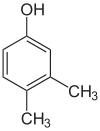
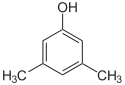
2,6-Xylenol 3,4-Xylenol 3,5-xylenol Functional group isomerism
Functional isomers are structural isomers that have the same molecular formula (that is, the same number of atoms of the same elements), but the atoms are connected together in different ways so that the groupings are dissimilar. These groups of atoms are called functional groups, functionalities, or moieties. Another way to say this is that two compounds with the same molecular formula, but different functional groups, are functional isomers.
For example, cyclohexane and 1-hexene both have the formula C6H12. These two are considered functional group isomers because cyclohexane is a cycloalkane and hex-1-ene is an alkene.
Example of functional group isomerism 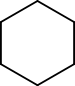
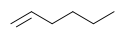
Cyclohexane 1-hexene For two molecules to be functional isomers, they must contain key groups of atoms arranged in particular ways. Some of the best examples come from organic chemistry. C2H6O is a molecular formula. Depending on how the atoms are arranged, it can represent two different compounds dimethyl ether CH3-O-CH3 or ethanol CH3CH2-O-H. Dimethyl ether and ethanol are functional isomers. The first is an ether. The carbon chain-oxygen-carbon chain functionality is called an ether. The second is an alcohol. The carbon chain-oxygen-hydrogen functionality is called an alcohol.
If the functionalities stay the same, but their locations change, the structural isomers are not functional isomers. 1-Propanol and 2-propanol are structural isomers, but they are not functional isomers. Both of them are alcohols. The functional group (carbon chain-O-H) is present in both of these compounds, but they are not the same.
While some chemists use the terms structural isomer and functional isomer interchangeably, not all structural isomers are functional isomers.
Functional isomers are most often identified in chemistry using infrared spectroscopy. Infrared radiation corresponds to the energies associated primarily with molecular vibration. The alcohol functionality has a very distinct vibration called OH-stretch that is due to hydrogen bonding. All alcohols in liquid and solid form absorb infrared radiation at certain wavelengths.
Compounds with the same functional groups will all absorb certain wavelengths of infrared light because of the vibrations associated with those groups. In fact, the infrared spectrum is divided into two regions. The first part is called the functional group region. Dimethyl ether and ethanol would have dissimilar infrared spectra in the functional group region.
The second part of the infrared spectrum is called the fingerprint region; it is associated with types of motion allowed by the symmetry of the molecule and influenced by the bond energies. The fingerprint region is more specific to an individual compound. Even though 1-propanol and 2-propanol have similar infrared spectra in the functional group region, they differ in the fingerprint region.
In simple terms, functional isomers are structural isomers that have different functional groups like alcohol and ether.
Isomer counting
As an example of isomer counting, 7 structural isomers exist with molecular formula C3H6O, each with different bond connectivities and air-stable at ambient temperature. An additional two structural isomers are the enol tautomers of the carbonyl isomers, but these are not stable.
Chemical compound Molecular structure Melting point (°C) Boiling point (°C) Comment Allyl alcohol 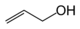
-129 97 Cyclopropanol 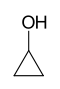
101–102 Propanal 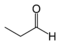
- 81 48 Tautomeric with (E)-1-propenol and (Z)-1-propenol Acetone 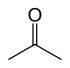
- 94.9 56.53 Tautomeric with 2-propenol Oxetane 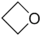
- 97 48 Propylene oxide 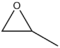
- 112 34 Can be resolved in two enantiomers Methyl vinyl ether 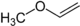
- 122 6 References
- ^ Clark, Jim. "Structural isomerism" in Chemguide, n.l., 2000, December 7 Web article
Categories:- Isomerism
Wikimedia Foundation. 2010.
