- Isopentane
-
Isopentane 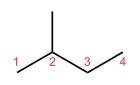
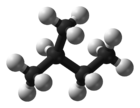 2-MethylbutaneOther namesMethylbutane
2-MethylbutaneOther namesMethylbutaneIdentifiers CAS number 78-78-4 
ChemSpider 6308 
UNII ZH67814I0O 
ChEBI CHEBI:30362 
RTECS number EK4430000 Jmol-3D images Image 1 - CC(C)CC
Properties Molecular formula C5H12 Molar mass 72.15 g/mol Appearance colorless liquid Density 0.616 g/ml, liquid[1] Melting point −159.9 °C (113.3 K)[1]
Boiling point 27.7 °C (300.9 K)[1]
Solubility in water Immiscible Thermochemistry Std enthalpy of
formation ΔfHo298−179 kJ/mol Std enthalpy of
combustion ΔcHo298−3504 kJ/mol Standard molar
entropy So298260.7 J·K−1·mol−1 Hazards EU classification Highly flammable (F+)
Harmful (Xn)
Dangerous for
the environment (N)R-phrases R12, R51/53, R65,
R66, R67S-phrases (S2), S9, S16, S29,
S33, S61, S62NFPA 704 Flash point <−51 °C Autoignition
temperature420 °C Explosive limits 1.4–7.6% Related compounds Related alkane Isobutane
Neopentane
2-MethylpentaneRelated compounds Pentane
Cyclopentane (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Isopentane, C5H12, also called methylbutane or 2-methylbutane, is a branched-chain alkane with five carbon atoms. Isopentane is an extremely volatile and extremely flammable liquid at room temperature and pressure. The normal boiling point is just a few degrees above room temperature and isopentane will readily boil and evaporate away on a warm day. Isopentane is commonly used in conjunction with liquid nitrogen to achieve a liquid bath temperature of -160 °C.
An isopentyl group is a subset of the generic pentyl group. It has the chemical structure -CH3CH2CH(CH3)2.
Contents
Nomenclature
Isopentane is the name recommended by the International Union of Pure and Applied Chemistry (IUPAC) in its 1993 Recommendations for the Nomenclature of Organic Chemistry.[2] It is one of only four acyclic hydrocarbons to retain its pre-IUPAC name. An isopentyl group is a subset of the generic pentyl group. It has the chemical structure -CH3CH2CH(CH3)2.
Isomers
Isopentane is one of three structural isomers with the molecular formula C5H12, the others being pentane (n-pentane) and dimethyl propane (neopentane).
Uses
Isopentane is one of the ingredients in both Aquafresh® and Sensodyne®.[3]
References
- ^ a b c James Wei (1999), Molecular Symmetry, Rotational Entropy, and Elevated Melting Points. Ind. Eng. Chem. Res., volume 38 issue 12, pp. 5019–5027 {{doi:10.1021/ie990588m}}
- ^ Panico, R.; & Powell, W. H. (Eds.) (1994). A Guide to IUPAC Nomenclature of Organic Compounds 1993. Oxford: Blackwell Science. ISBN 0-632-03488-2. http://www.acdlabs.com/iupac/nomenclature/93/r93_679.htm.
- ^ Aquafresh Website
External links
- International Chemical Safety Card 1153
- IUPAC Nomenclature of Organic Chemistry (online version of the "Blue Book")
Alkanes Higher alkanes · List of alkanes Categories:- Alkanes
Wikimedia Foundation. 2010.

