- Isobutane
-
Isobutane 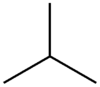
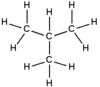
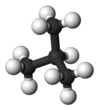
 isobutane2-methylpropaneOther namesmethylpropane
isobutane2-methylpropaneOther namesmethylpropaneIdentifiers CAS number 75-28-5 
PubChem 6360 ChemSpider 6120 
UNII BXR49TP611 
ChEBI CHEBI:30363 
Jmol-3D images Image 1 - CC(C)C
Properties Molecular formula C4H10 Molar mass 58.12 g mol−1 Appearance colorless gas Density 2.51 kg/m3, gas (15 °C, 1 atm)
593.4 kg/m3, liquidMelting point -159.6 °C, 114 K, -255 °F
Boiling point -11.7 °C, 261 K, 11 °F
Solubility in water Insoluble Hazards MSDS External MSDS EU classification Highly flammable (F+) R-phrases R12 S-phrases (S2), S9, S16 NFPA 704 Flash point flammable gas Autoignition
temperature460 °C Explosive limits 1.8–8.4% Related compounds Related alkane Butane Related compounds Isopentane
NeopentaneSupplementary data page Structure and
propertiesn, εr, etc. Thermodynamic
dataPhase behaviour
Solid, liquid, gasSpectral data UV, IR, NMR, MS  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Isobutane, also known as methylpropane, is an isomer of butane. It is the simplest alkane with a tertiary carbon. Concerns with depletion of the ozone layer by freon gases have led to increased use of isobutane as a gas for refrigeration systems, especially in domestic refrigerators and freezers, and as a propellant in aerosol sprays. When used as a refrigerant or a propellant, isobutane is also known as R-600a. Some portable camp stoves use a mixture of isobutane with propane, usually 80:20. Isobutane is used as a feedstock in the petrochemical industry, for example in the synthesis of isooctane.[1]
Its UN number (for hazardous substances see shipping) is UN 1969. Isobutane is the R group for the amino acid leucine.Contents
Nomenclature
 Structures of the two isomers of butane
Structures of the two isomers of butane
Isobutane is the trivial name retained by the International Union of Pure and Applied Chemistry (IUPAC) in its 1993 Recommendations for the Nomenclature of Organic Chemistry.[2] Since the longest continuous chain in isobutane is only three carbon atoms, the systematic name is methylpropane. The position number (2-) is unnecessary because it is the only possibility in methylpropane.
Uses
- As a refrigerant.[3]
The use in refrigerators started in 1993 when Greenpeace presented the Greenfreeze project with the German company Foron.[4]
Blends of pure, dry "isobutane" (R-600a) (commercial term used to describe isobutane mixtures) have negligible ozone depletion potential and very low Global Warming Potential (having a value of 3.3 times the GWP of carbon dioxide) and can serve as a functional replacement for R-12, R-22, R-134a, and other chlorofluorocarbon or hydrofluorocarbon refrigerants in conventional stationary refrigeration and air conditioning systems.
- As a propellant for aerosol cans and foam products.
Safety Concerns
Reports surfaced in late 2009 suggesting the use of isobutane as a refrigerant in domestic refrigerators was potentially dangerous. Several explosions resulting from the isobutane leaking into the refrigerator cabinet and a spark from the electrical system have been reported in the United Kingdom.[5] Although unclear how serious this could be, at the time this report came out it was estimated 300 million refrigerators worldwide use isobutane as a refrigerant.
Although these stories are only speculation, the use of a flammable gas as a refrigerant is quite dangerous and encompasses a great deal of risk. The normal risks a CFC or other toxic refrigerant would have when it escapes, are mainly related to depletion of breathable air and frosting at the point of escape. Isobutane has an explosion risk associated also (in addition to depletion of breathable air and frosting). This explosion risk is more dangerous directly to anyone in the vicinity should it accumulate and come into contact with any ignition source. To reduce the risk, the charge of refrigerators was reduced by more than 50% against R134a. The typical charge of a R600a freezer is 45g and of a refrigerator 20g. If 45g are lost at once, 1m³ of explosive gas can be created. The risk that all gas is lost outside in a short time is very low. For interior refrigeration applications, the R600a appliances should have a hidden condenser, so that the gas can not be lost. 20g is for example approximately 3-4 times the charge of a cigarette lighter. In Europe the limit is 150g for R600a applinces due to safety.
According to an MSDS (Material Safety Data Sheet) for isobutane, this gas should not be exposed to temperatures above 52 Celsius or 151 Fahrenheit while in a closed system such as a storage tank.[6] The range of flammability also factors into safety of this substance as a refrigerant. Flammable ranges for isobutane are between 1.8% - 8.4% and this creates a hazard when a leak forms in the refrigeration system. Many sources of ignition may be nearby so the unit could possibly explode due to the gas leak and cause major damage, not to mention injuries or death to persons nearby. This will create a problem for refrigeration technicians if a leak is reported. The leak must be checked in open air or well ventilated environments and the technician could risk injury to themselves or others. In essence, the refrigeration unit would need full replacement in most cases.
Substitution of this refrigerant for motor vehicle air conditioning systems not originally designed for R600a is widely prohibited or discouraged, on the grounds that using flammable hydrocarbons in systems originally designed to carry non-flammable refrigerant presents a significant risk of fire or explosion.[7][8][9][10][11][12][13][14]
Vendors and advocates of hydrocarbon refrigerants argue against such bans on the grounds that there have been very few such incidents relative to the number of vehicle air conditioning systems filled with hydrocarbons.[15][16] One particular test was conducted by a professor at the University of New South Wales that unintentionally tested the scenario of a sudden and complete refrigerant loss into the passenger compartment followed by subsequent ignition. He and several others in the car sustained burns to their face, ears, and hands, and several observers received lacerations from the burst glass of the front passenger window.[17]
References
- ^ Patent Watch, July 31, 2006.
- ^ Panico, R.; & Powell, W. H. (Eds.) (1994). A Guide to IUPAC Nomenclature of Organic Compounds 1993. Oxford: Blackwell Science. ISBN 0-632-03488-2. http://www.acdlabs.com/iupac/nomenclature/93/r93_679.htm
- ^ "European Commission on retrofit refrigerants for stationary applications" (PDF). http://ec.europa.eu/environment/ozone/pdf/hcfc_technical_meeting_summary.pdf. Retrieved 2010-10-29.
- ^ http://www.greenpeace.org/usa/en/campaigns/global-warming-and-energy/green-solutions/greenfreeze/
- ^ Bingham, John (September 1, 2009). "Exploding fridges: ozone friendly gas theory for mystery blasts". The Daily Telegraph (London). http://www.telegraph.co.uk/news/picturegalleries/howaboutthat/6120297/Exploding-fridges-ozone-friendly-gas-theory-for-mystery-blasts.html. Retrieved May 5, 2010.
- ^ http://www.praxair.com/praxair.nsf/24116e7e98e6297f8525705a006f3694/b7e46554268d4c55852575bc0067d5b6/$FILE/p4613d.pdf
- ^ "U.S. EPA hydrocarbon-refrigerants FAQ". Epa.gov. http://www.epa.gov/ozone/snap/refrigerants/hc12alng.html. Retrieved 2010-10-29.
- ^ Compendium of hydrocarbon-refrigerant policy statements, October 2006[dead link]
- ^ "MACS bulletin: hydrocarbon refrigerant usage in vehicles" (PDF). http://www.autoacforum.com/MACS/HCwarning.pdf. Retrieved 2010-10-29.
- ^ "Society of Automotive Engineers hydrocarbon refrigerant bulletin". Sae.org. 2005-04-27. http://www.sae.org/news/releases/05hydrocarbon_warning.htm. Retrieved 2010-10-29.
- ^ "Shade Tree Mechanic on hydrocarbon refrigerants". Shadetreemechanic.com. 2005-04-27. http://www.shadetreemechanic.com/cc_hydrocarbon_refrigerants.htm. Retrieved 2010-10-29.
- ^ "Saskatchewan Labour bulletin on hydrocarbon refrigerants in vehicles". Labour.gov.sk.ca. 2010-06-29. http://www.labour.gov.sk.ca/Default.aspx?DN=2fb5ac24-d90e-4408-bf40-559793bd8e96. Retrieved 2010-10-29.
- ^ VASA on refrigerant legality & advisability[dead link]
- ^ "Queensland (Australia) government warning on hydrocarbon refrigerants". Energy.qld.gov.au. http://www.energy.qld.gov.au/zone_files/petroleum_pdf/safety_alert025.pdf. Retrieved 2010-10-29.
- ^ "New South Wales (Australia) Parliamentary record, 16 October 1997". Parliament.nsw.gov.au. 1997-10-16. http://www.parliament.nsw.gov.au/prod/parlment/HansArt.nsf/V3Key/LA19971016015. Retrieved 2010-10-29.
- ^ "New South Wales (Australia) Parliamentary record, 29 June 2000". Parliament.nsw.gov.au. http://www.parliament.nsw.gov.au/prod/parlment/hansart.nsf/V3Key/LC20000629051. Retrieved 2010-10-29.
- ^ VASA news report on hydrocarbon refrigerant demonstrations[dead link]
External links
- International Chemical Safety Card 0901
- NIOSH Pocket Guide to Chemical Hazards
- IUPAC Nomenclature of Organic Chemistry (online version of the "Blue Book")
- Data from Air Liquide
E numbers Colors (E100–199) · Preservatives (E200–299) · Antioxidants & acidity regulators (E300–399) · Thickeners, stabilisers & emulsifiers (E400–499) · pH regulators & anti-caking agents (E500–599) · Flavour enhancers (E600–699) · Miscellaneous (E900–999) · Additional chemicals (E1100–1599)
Waxes (E900–909) · Synthetic glazes (E910–919) · Improving agents (E920–929) · Packaging gases (E930–949) · Sweeteners (E950–969) · Foaming agents (E990–999)
Calcium peroxide (E930) · Argon (E938) · Helium (E939) · Dichlorodifluoromethane (E940) · Nitrogen (E941) · Nitrous oxide (E942) · Butane (E943a) · Isobutane (E943b) · Propane (E944) · Oxygen (E948) · Hydrogen (E949)
Categories:- Alkanes
- Refrigerants
- Propellants
Wikimedia Foundation. 2010.

