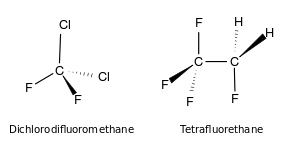
- Chlorofluorocarbon
-
A chlorofluorocarbon (CFC) is an organic compound that contains carbon, chlorine, and fluorine, produced as a volatile derivative of methane and ethane. A common subclass are the hydrochlorofluorocarbons (HCFCs), which contain hydrogen, as well. They are also commonly known by the DuPont trade name Freon. The most common representative is dichlorodifluoromethane (R-12 or Freon-12). Many CFCs have been widely used as refrigerants, propellants (in aerosol applications), and solvents. The manufacture of such compounds has been phased out by the Montreal Protocol because they contribute to ozone depletion.
Contents
Structure, properties, production
Main article: Organofluorine chemistryAs in simpler alkanes, carbon in the CFCs and the HCFCs is tetrahedral. Since the fluorine and chlorine atoms differ greatly in size from hydrogen and from each other, the methane derived CFCs deviate from perfect tetrahedral symmetry.[1]
The physical properties of the CFCs and HCFCs are tunable by changes in the number and identity of the halogen atoms. In general they are volatile, but less so than parent alkane. The decreased volatility is attributed to the molecular polarity induced by the halides and the polarizability of halides, which induces intermolecular interactions. Thus, methane boils at −161 °C whereas the fluoromethanes boil between −51.7 (CF2H2) and −128 °C (CF4). The CFCs have still higher boiling points because the chloride is even more polarizable than fluoride. Because of their polarity, the CFCs are useful solvents. The CFCs are far less flammable than methane, in part because they contain fewer C-H bonds and in part because, in the case of the chlorides and bromides, the released halides quench the free radicals that sustain flames.
The densities of CFCs are invariably higher than the corresponding alkanes. In general the density of these compounds correlates with the number of chlorides.
CFCs and HCFCs are usually produced by halogen exchange starting from chlorinated methanes and ethanes. Illustrative is the synthesis of chlorodifluoromethane from chloroform:
- HCCl3 + 2 HF → HCF2Cl + 2 HCl
The brominated derivatives are generated by free-radical reactions of the chlorofluorocarbons, replacing C-H bonds with C-Br bonds. The production of the anesthetic 2-bromo-2-chloro-1,1,1-trifluoroethane ("halothane") is illustrative:
- CF3CH2Cl + Br2 → CF3CHBrCl + HBr
Reactions
The most important reaction of the CFCs is the photo-induced scission of a C-Cl bond:
- CCl3F → CCl2F. + Cl.
The chlorine atom, written often as Cl., behaves very differently from the chlorine molecule (Cl2). The radical Cl. is long-lived in the upper atmosphere, where it catalyzes the conversion of ozone into O2. Ozone absorbs UV-radiation better than O2 does, so its depletion allows more of this high energy radiation to reach the Earth's surface. Bromine atoms are even more efficient catalysts, hence brominated CFCs are also regulated.
Applications
Applications exploit the low toxicity, low reactivity, and low flammability of the CFCs and HCFCs. Every permutation of fluorine, chlorine, and hydrogen based on methane and ethane has been examined and most have been commercialized. Furthermore, many examples are known for higher numbers of carbon as well as related compounds containing bromine. Uses include refrigerants, blowing agents, propellants in medicinal applications, and degreasing solvents.
Billions of kilograms of chlorodifluoromethane are produced annually as a precursor to tetrafluoroethylene, the monomer that is converted into Teflon.[2]
Classes of compounds, nomenclature
- Chlorofluorocarbons (CFCs): when derived from methane and ethane these compounds have the formulae CClmF4-m and C2ClmF6-m, where m is nonzero.
- Hydrochlorofluorocarbons (HCFCs): when derived from methane and ethane these compounds have the formulae CClmFnH4-m-n and C2ClxFyH6-x-y, where m, n, x, and y are nonzero.
- Bromochlorofluorocarbons and bromofluorocarbons have formulae similar to the CFCs and HCFCs but also bromine.
- Hydrofluorocarbons (HFC's): when derived from methane, ethane, propane, and butane, these compounds have the respective formulae CFmH4-m, C2FmH6-m, C3FmH8-m, and C4FmH10-m, where m is nonzero.
Commercial names
Freon is DuPont's brand name for CFCs, HCFCs and related compounds. Other commercial names from around the world are Algofrene, Arcton, Asahiflon, Daiflon, Eskimo, FCC, Flon, Flugene, Forane, Fridohna, Frigen, Frigedohn, Genetron, Isceon, Isotron, Kaiser, Kaltron, Khladon, Ledon, Racon, and Ucon.
Numbering system
Main article: refrigerant#Refrigerants by classA numbering system is used for fluorinated alkanes, prefixed with Freon-, R-, CFC-, and HCFC-. The rightmost value indicates the number of fluorine atoms, the next value to the left is the number of hydrogen atoms plus 1, and the next value to the left is the number of carbon atoms less one (zeroes are not stated). Remaining atoms are chlorine. Thus, Freon-12 indicates a methane derivative (only two numbers) containing two fluorine atoms (the second 2) and no hydrogen (1-1=0). It is therefore CCl2F2.
Another, easier equation that can be applied to get the correct molecular formula of the CFC/R/Freon class compounds is this to take the numbering and add 90 to it. The resulting value will give the number of carbons as the first numeral, the second numeral gives the number of hydrogen atoms, and the third numeral gives the number of fluorine atoms. The rest of the unaccounted carbon bonds are occupied by chlorine atoms. The value of this equation is always a three figure number. An easy example is that of CFC-12, which gives: 90+12=102 -> 1 carbon, 0 hydrogens, 2 fluorine atoms, and hence 2 chlorine atoms resulting in CCl2F2. The main advantage of this method of deducing the molecular composition in comparison with the method described in the paragraph above, is that it gives the number of carbon atoms of the molecule.
Freons containing bromine is signified by four numbers. Isomers, which are common for ethane and propane derivatives, are indicated by letters following the numbers.
Principal CFCs Systematic name Common/trivial
name(s), codeBoiling point (°C) Formula Trichlorofluoromethane Freon-11, R-11, CFC-11 23 CCl3F Dichlorodifluoromethane Freon-12, R-12, CFC-12 −29.8 CCl2F2 Chlorotrifluoromethane Freon-13, R-13, CFC-13 −81 CClF3 Chlorodifluoromethane R-22, HCFC-22 −40.8 CHClF2 Dichlorofluoromethane R-21, HCFC-21 8.9 CHCl2F Chlorofluoromethane Freon 31, R-31, HCFC-31 CH2ClF Bromochlorodifluoromethane BCF, Halon 1211, H-1211, Freon 12B1 CBrClF2 1,1,2-Trichloro-1,2,2-trifluoroethane Freon 113, R-113, CFC-113, 1,1,2-Trichlorotrifluoroethane 47.7 Cl2FC-CClF2 1,1,1-Trichloro-2,2,2-trifluoroethane Freon 113a, R-113a, CFC-113a 45.9 Cl3C-CF3 1,2-Dichloro-1,1,2,2-tetrafluoroethane Freon 114, R-114, CFC-114, Dichlorotetrafluoroethane 3.8 ClF2C-CClF2 1-Chloro-1,1,2,2,2-pentafluoroethane Freon 115, R-115, CFC-115, Chloropentafluoroethane −38 ClF2C-CF3 2-Chloro-1,1,1,2-tetrafluoroethane R-124, HCFC-124 −12 CHFClCF3 1,1-Dichloro-1-fluoroethane R-141b, HCFC-141b 32 Cl2FC-CH3 1-Chloro-1,1-difluoroethane R-142b, HCFC-142b −9.2 ClF2C-CH3 Tetrachloro-1,2-difluoroethane Freon 112, R-112, CFC-112 91.5 CCl2FCCl2F Tetrachloro-1,1-difluoroethane Freon 112a, R-112a, CFC-112a 91.5 CClF2CCl3 1,1,2-Trichlorotrifluoroethane Freon 113, R-113, CFC-113 48 CCl2FCClF2 1-Bromo-2-chloro-1,1,2-trifluoroethane Halon 2311a 51.7 CHClFCBrF2 2-Bromo-2-chloro-1,1,1-trifluoroethane Halon 2311 50.2 CF3CHBrCl 1,1-Dichloro-2,2,3,3,3-pentafluoropropane R-225ca, HCFC-225ca 51 CF3CF2CHCl2 1,3-Dichloro-1,2,2,3,3-pentafluoropropane R-225cb, HCFC-225cb 56 CClF2CF2CHClF History
Carbon tetrachloride (CCl4) was used in fire extinguishers and glass "anti-fire grenades" from the late nineteenth century until around the end of World War II. Experimentation with chloroalkanes for fire suppression on military aircraft began at least as early as the 1920s. Freon is a trade name for a group of CFCs which are used primarily as refrigerants, but also have uses in fire-fighting and as propellants in aerosol cans. Bromomethane is widely used as a fumigant. Dichloromethane is a versatile industrial solvent.
The Belgian scientist Frédéric Swarts pioneered the synthesis of CFCs in the 1890s. He developed an effective exchange agent to replace chloride in carbon tetrachloride with fluoride to synthesize CFC-11 (CCl3F) and CFC-12 (CCl2F2).
In the late 1920s, Thomas Midgley, Jr. improved the process of synthesis and led the effort to use CFC as refrigerant to replace ammonia (NH3), chloromethane (CH3Cl), and sulfur dioxide (SO2), which are toxic but were in common use. In searching for a new refrigerant, requirements for the compound were: low boiling point, low toxicity, and to be generally non-reactive. In a demonstration for the American Chemical Society, Midgley flamboyantly demonstrated all these properties by inhaling a breath of the gas and using it to blow out a candle[3] in 1930.[4][5]
During World War II, various chloroalkanes were in standard use in military aircraft, although these early halons suffered from excessive toxicity. Nevertheless, after the war they slowly became more common in civil aviation as well. In the 1960s, fluoroalkanes and bromofluoroalkanes became available and were quickly recognized as being highly effective fire-fighting materials. Much early research with Halon 1301 was conducted under the auspices of the US Armed Forces, while Halon 1211 was, initially, mainly developed in the UK. By the late 1960s they were standard in many applications where water and dry-powder extinguishers posed a threat of damage to the protected property, including computer rooms, telecommunications switches, laboratories, museums and art collections. Beginning with warships, in the 1970s, bromofluoroalkanes also progressively came to be associated with rapid knockdown of severe fires in confined spaces with minimal risk to personnel.
By the early 1980s, bromofluoroalkanes were in common use on aircraft, ships, and large vehicles as well as in computer facilities and galleries. However, concern was beginning to be expressed about the impact of chloroalkanes and bromoalkanes on the ozone layer. The Vienna Convention for the Protection of the Ozone Layer did not cover bromofluoroalkanes as it was thought, at the time, that emergency discharge of extinguishing systems was too small in volume to produce a significant impact, and too important to human safety for restriction.
Regulation
Since the late 1970s, the use of CFCs has been heavily regulated because of their destructive effects on the ozone layer. After the development of his electron capture detector, James Lovelock was the first to detect the widespread presence of CFCs in the air, finding a mole fraction of 60 ppt of CFC-11 over Ireland. In a self-funded research expedition ending in 1973, Lovelock went on to measure CFC-11 in both the Arctic and Antarctic, finding the presence of the gas in each of 50 air samples collected, and concluding that CFCs are not hazardous to the environment. The experiment did however provide the first useful data on the presence of CFCs in the atmosphere. The damage caused by CFCs was discovered by Sherry Rowland and Mario Molina who, after hearing a lecture on the subject of Lovelock's work, embarked on research resulting in the first publication suggesting the connection in 1974. It turns out that one of CFCs' most attractive features—their low reactivity— is key to their most destructive effects. CFCs' lack of reactivity gives them a lifespan that can exceed 100 years, giving them time to diffuse into the upper stratosphere. Once in the stratosphere, the sun's ultraviolet radiation is strong enough to cause the homolytic cleavage of the C-Cl bond.
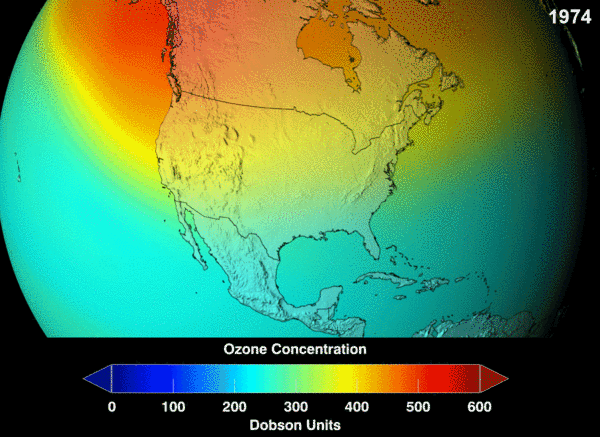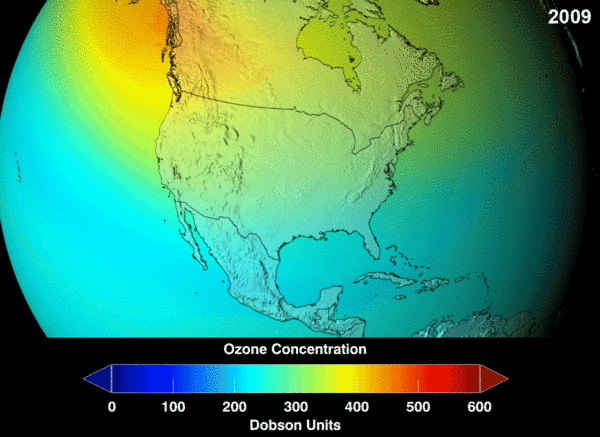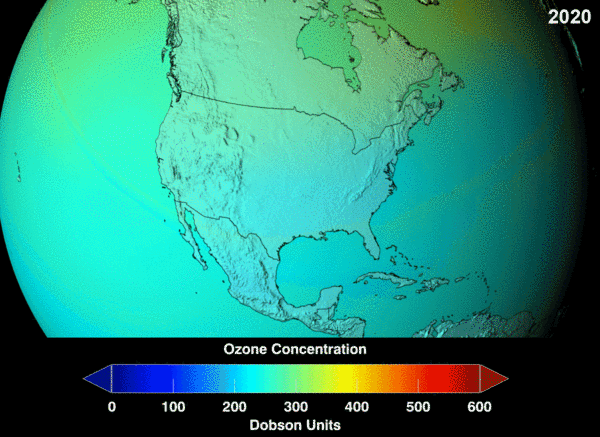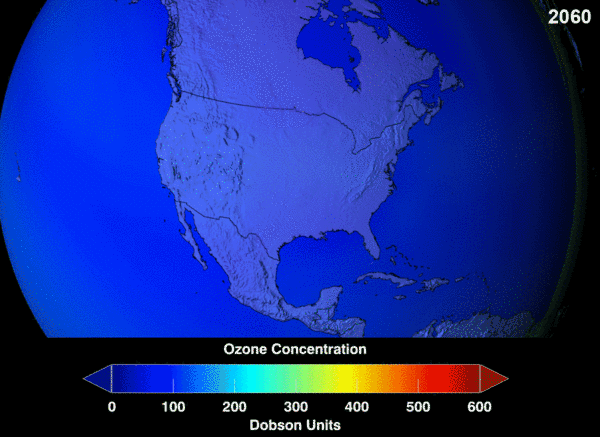 NASA projection of stratospheric ozone, in Dobson units, if chlorofluorocarbons had not been banned.
NASA projection of stratospheric ozone, in Dobson units, if chlorofluorocarbons had not been banned.
By 1987, in response to a dramatic seasonal depletion of the ozone layer over Antarctica, diplomats in Montreal forged a treaty, the Montreal Protocol, which called for drastic reductions in the production of CFCs. On March 2, 1989, 12 European Community nations agreed to ban the production of all CFCs by the end of the century. In 1990, diplomats met in London and voted to significantly strengthen the Montreal Protocol by calling for a complete elimination of CFCs by the year 2000. By the year 2010 CFCs should be completely eliminated from developing countries as well.
Because the only CFCs available to countries adhering to the treaty is from recycling, their prices have increased considerably. A worldwide end to production should also terminate the smuggling of this material. However, there are current CFC smuggling issues, as recognized by UNEP (United Nations Environmental Programme)in a 2006 report titled "Illegal Trade in Ozone Depleting Substances". UNEP estimates that between 16,000–38,000 tonnes of CFCs passed through the black market in the mid-1990s. The report estimated between 7,000 and 14,000 tonnes of CFCs are smuggled annually into developing countries. Asian countries are those with the most smuggling; China, India and South Korea were found to account for around 70% of global CFC production.[6] Possible reasons for continued CFC smuggling were also examined: the report noted that many banned CFC producing products have long lifespans and continue to operate. The cost of replacing the equipment of these items is sometimes cheaper than outfitting them with a more ozone-friendly appliance. Additionally, CFC smuggling is not considered a significant issue so the perceived penalties for smuggling are low. While the eventual phaseout of CFCs is likely, efforts are being taken to stem these current non-compliance problems.
By the time of the Montreal Protocol it was realised that deliberate and accidental discharges during system tests and maintenance accounted for substantially larger volumes than emergency discharges, and consequently halons were brought into the treaty, albeit with many exceptions.
Regulatory gap
While the production and consumption of CFCs are regulated under the Montreal Protocol, emissions from pre-existing banks of CFCs are not regulated under the agreement. As of 2002, there were 5,791 kilotons of CFCs in existing products such as refrigerators, air conditioners, aerosol cans and others.[7] Approximately one-third of these CFCs are projected to be emitted over the next decade if action is not taken, posing a threat to both the ozone layer and the climate.[8] A proportion of these CFCs can be safely captured and destroyed.
Regulation and DuPont
In 1978 the United States banned the use of CFCs such as Freon in aerosol cans, the beginning of a long series of regulatory actions against their use. The critical DuPont manufacturing patent for Freon ("Process for Fluorinating Halohydrocarbons", U.S. Patent #3258500) was set to expire in 1979. In conjunction with other industrial peers DuPont sponsored efforts such as the "Alliance for Responsible CFC Policy" to question anti-CFC science, but in a turnabout in 1986 DuPont, with new patents in hand, publicly condemned CFCs.[9] DuPont representatives appeared before the Montreal Protocol urging that CFCs be banned worldwide and stated that their new HCFCs would meet the worldwide demand for refrigerants.[9]
Phase out of CFCs
Use of certain chloroalkanes as solvents for large scale application, such as dry cleaning, have been phased out, for example, by the IPPC directive on greenhouse gases in 1994 and by the Volatile Organic Compounds (VOC) directive of the EU in 1997. Permitted chlorofluoroalkane uses are medicinal only.
Bromofluoroalkanes have been largely phased out and the possession of equipment for their use is prohibited in some countries like the Netherlands and Belgium, from 1 January 2004, based on the Montreal Protocol and guidelines of the European Union.
Production of new stocks ceased in most (probably all) countries as of 1994.[citation needed] However many countries still require aircraft to be fitted with halon fire suppression systems because no safe and completely satisfactory alternative has been discovered for this application. There are also a few other, highly specialized uses. These programs recycle halon through "halon banks" coordinated by the Halon Recycling Corporation[10] to ensure that discharge to the atmosphere occurs only in a genuine emergency and to conserve remaining stocks.
The interim replacements for CFCs are hydrochlorofluorocarbons (HCFCs), which deplete stratospheric ozone, but to a much lesser extent than CFCs.[11] Ultimately, hydrofluorocarbons (HFCs) will replace HCFCs. Unlike CFCs and HCFCs, HFCs have a ozone depletion potential (ODP) of 0. (although all three groups of halocarbons are powerful greenhouse gases). DuPont began producing hydrofluorocarbons as alternatives to Freon in the 1980s. These included Suva refrigerants and Dymel propellants.[12] Natural refrigerants are climate friendly solutions that are enjoying increasing support from large companies and governments interested in reducing global warming emissions from refrigeration and air conditioning. Hydrofluorocarbons are included in the Kyoto Protocol because of their very high Global Warming Potential and are facing calls to be regulated under the Montreal Protocol[dubious ][13] due to the recognition of halocarbon contributions to climate change.[14]
On September 21, 2007, approximately 200 countries agreed to accelerate the elimination of hydrochlorofluorocarbons entirely by 2020 in a United Nations-sponsored Montreal summit. Developing nations were given until 2030. Many nations, such as the United States and China, who had previously resisted such efforts, agreed with the accelerated phase out schedule;[15] however, presently China and Brazil are nations notable for their large and increasing production of chlorofluorocarbons.[16]
Development of alternatives for CFCs
Work on alternatives for chlorofluorocarbons in refrigerants began in the late 1970s after the first warnings of damage to stratospheric ozone were published. The hydrochlorofluorocarbons (HCFCs) are less stable in the lower atmosphere, enabling them to break down before reaching the ozone layer. Nevertheless, a significant fraction of the HCFCs do break down in the stratosphere and they have contributed to more chlorine buildup there than originally predicted. Later alternatives lacking the chlorine, the hydrofluorocarbons (HFCs) have an even shorter lifetimes in the lower atmosphere. One of these compounds, HFC-134a, is now used in place of CFC-12 in automobile air conditioners. Hydrocarbon refrigerants (a propane/isobutane blend) are also used extensively in mobile air conditioning systems in Australia, the USA and many other countries, as they have excellent thermodynamic properties and perform particularly well in high ambient temperatures. One of the natural refrigerants (along with Ammonia and Carbon Dioxide), hydrocarbons have negligible environmental impacts and are also used worldwide in domestic and commercial refrigeration applications, and are becoming available in new split system air conditioners.[17] Various other solvents and methods have replaced the use of CFCs in laboratory analytics.[18]
Applications and replacements for CFCs Application Previously used CFC Replacement Refrigeration & air-conditioning CFC-12 (CCl2F2); CFC-11(CCl3F); CFC-13(CClF3); HCFC-22 (CHClF2); CFC-113 (Cl2FCCClF2); CFC-114 (CClF2CClF2); CFC-115 (CF3CClF2); HFC-23 (CHF3); HFC-134a (CF3CFH2); HFC-507 (a 1:1 azeotropic mixture of HFC 125 (CF3 CHF2) and HFC-143a (CF3CH3)); HFC 410 (a 1:1 azeotropic mixture of HFC-32 (CF2H2) and HFC-125 (CF3CF2H)) Propellants in medicinal aerosols CFC-114 (CClF2CClF2) HFC-134a (CF3CFH2); HFC-227ea (CF3CHFCF3) Blowing agents for foams CFC-11 (CCl3F); CFC 113 (Cl2FCCClF2); HCFC-141b (CCl2FCH3) HFC-245fa (CF3CH2CHF2); HFC-365 mfc (CF3CH2CF2CH3) Solvents, degreasing agents, cleaning agents CFC-11 (CCl3F); CFC-113 (CCl2FCClF2) None Environmental impacts
As previously discussed, CFCs were phased out via the Montreal Protocol due to their part in ozone depletion. However, the atmospheric impacts of CFCs are not limited to its role as an active ozone reducer. This anthropogenic compound is also a greenhouse gas, with a much higher potential to enhance the greenhouse effect than CO2.
Infrared bands trap heat from escaping earth's atmosphere. In the case of CFCs, the strongest of these bands are located at the spectral region – referred to as an atmospheric window due to the relative transparency of the atmosphere within this region.[19] The strength of CFC bands and the unique susceptibility of the atmosphere, at which the compound absorbs and emits radiation, are two factors that contribute to CFC's "super" greenhouse effect.[20] Another such factor is the low concentration of the compound. Because CO2 is close to saturation with high concentrations, it takes more of the substance to enhance the greenhouse effect. Conversely, the low concentration of CFCs allow their effects to increase linearly with mass.[20]
Tracer of ocean circulation
Since the time history of CFC concentrations in the atmosphere is relatively well known, they have provided an important constraint on ocean circulation. CFCs dissolve in seawater at the ocean surface and are subsequently transported into the ocean interior. Since CFCs are conservative, their concentration in the ocean interior reflects simply the convolution of their atmospheric time evolution and ocean circulation and mixing.
Safety
According to their material safety data sheets, CFCs and HCFCs are colourless, volatile, toxic liquids and gases with a faintly sweet ethereal odour. Overexposure may cause dizziness, loss of concentration, central nervous system depression and/or cardiac arrhythmia. Vapors displace air and can cause asphyxiation in confined spaces. Although non-flammable, their combustion products include hydrofluoric acid, and related species.[21]
References
- ^ Günter Siegemund, Werner Schwertfeger, Andrew Feiring, Bruce Smart, Fred Behr, Herward Vogel, Blaine McKusick “Fluorine Compounds, Organic” Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2002. doi:10.1002/14356007.a11_349
- ^ M. Rossberg et al. “Chlorinated Hydrocarbons” in Ullmann’s Encyclopedia of Industrial Chemistry 2006, Wiley-VCH, Weinheim. doi:10.1002/14356007.a06_233.pub2
- ^ Inventors accessed December 21, 2007
- ^ Carlisle, Rodney (2004). Scientific American Inventions and Discoveries, p.351. John Wiley & Songs, Inc., New Jersey. ISBN 0-471-24410-4.
- ^ McNeill, J.R. Something New Under the Sun: An Environmental History of the Twentieth-Century World (2001) New York: Norton, xxvi, 421 pp. (as reviewed in the Journal of Political Ecology)
- ^ "Illegal Trade in Ozone Depleting Substances." UNEP, 2007. Web. 3 Apr. 2011..
- ^ IPCC/TEAP Special Report on Ozone and Climate
- ^ Chlorofluorocarbons: An Overlooked Climate Threat, EESI Congressional Briefing. Eesi.org. Retrieved on 2011-09-24.
- ^ a b "Ethics of Du Pont's CFC Strategy 1975–1995", Smith B. Journal of Business Ethics, Volume 17, Number 5, April 1998, pp. 557–568(12)
- ^ Welcome to the Halon Corporation. Halon.org. Retrieved on 2011-09-24.
- ^ "Ozone Layer Depletion", U.S. Environmental Protection Agency accessed June 25, 2008
- ^ Freon® : 1930. In Depth. dupont.com (2009-01-30). Retrieved on 2011-09-24.
- ^ "A Novel Tactic in Climate Fight Gains Some Traction", ''NY Times''. Nytimes.com. Retrieved on 2011-09-24.
- ^ Velders et.al., PNAS March 20, 2007 vol. 104 no. 12 4814–4819 [1] "The importance of the Montreal Protocol in protecting climate"
- ^ HCFC Phaseout Schedule. Epa.gov (2006-06-28). Retrieved on 2011-09-24.
- ^ David Reay and C.Michael Hogan. 2011. Greenhouse gas. eds. Howard Hanson, Michael Pidwirny, C.J.Cleveland Encyclopedia of Earth. National Council for Science and the Environment. Washington DC
- ^ "Greenpeace, Cool Technologies". (PDF) . Retrieved on 2011-09-24.
- ^ Use of Ozone Depleting Substances in Laboratories. TemaNord 516/2003. Norden.org (2003-01-01). Retrieved on 2011-09-24.
- ^ Ramanathan, V (1975). "Greenhouse Effect Due to Chlorofluorocarbons: Climatic Implications". Science, New Series 19: 50–52. JSTOR 1740877.
- ^ a b Ramanathan, V; Y. Feng (2009). "Air pollution, greenhouse gases and climate change: Global and regional perspectives". Atmospheric Environment 43: 37–50. doi:10.1016/j.atmosenv.2008.09.063.
- ^ Material Safety Data Sheet. National Refrigerants
External links
- Gas conversion table
- Nomenclature FAQ
- Numbering Scheme for Ozone-Depleting Substances and their Substitutes
- Class I Ozone-Depleting Substances
- CFC Illegal Trade
- Numbering Scheme for Ozone-Depleting Substances and their Substitutes
- Class I Ozone-Depleting Substances
- Class II Ozone-Depleting Substances (HCFCs)
- History of Halon use by the US Navy
- Ozone Loss: The Chemical Culprits
- Process using pyrolysis in an ultra high temperature plasma arc for the elimination of CFCs
- [2] Environmental Investigation Agency: Reports etc. on illegal trade and solutions.
- [3] Environmental Investigation Agency in the USA: Reports etc. on illegal trade and solutions.
- Freon in Car A/C
Halomethanes Monosubstituted Disubstituted Trisubstituted Functional groups Acetyl · Acetoxy · Acryloyl · Acyl · Alcohol · Aldehyde · Alkane · Alkene · Alkyne · Alkoxy group · Amide · Amine · Azo compound · Benzene derivative · Carboxylic acid · Cyanate · Disulfide · Ester · Ether · Epoxide · Haloalkane · Hydrazone · Hydroxyl · Imine · Isocyanate · Isonitrile · Isothiocyanate · Ketone · Methine · Nitrile · Nitro compound · Nitroso compound · Organophosphorus · Oxime · Peroxide · Phosphonous and Phosphonic acid · Pyridine derivative · Sulfone · Sulfonic acid · Sulfoxide · Thiocyanate · Thioester · Thioether · Thiol · Urea
See also Chemical classificationCategories:- Aerosol propellants
- DuPont
- Firefighting
- Greenhouse gases
- Halogenated solvents
- Halomethanes
- Heating, ventilating, and air conditioning
- Ozone depletion
- Refrigerants
- Chlorofluorocarbons
Wikimedia Foundation. 2010.