- Trifluoroiodomethane
-
Trifluoroiodomethane 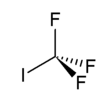
 TrifluoroiodomethaneOther namesIodotrifluoromethane, Monoiodotrifluoromethane, Trifluoromethyl iodide, Perfluoromethyl iodide, Freon 13T1
TrifluoroiodomethaneOther namesIodotrifluoromethane, Monoiodotrifluoromethane, Trifluoromethyl iodide, Perfluoromethyl iodide, Freon 13T1Identifiers CAS number 2314-97-8 
PubChem 16843 ChemSpider 15962 
UNII 42A379KB0U 
EC number 219-014-5 RTECS number PB6975000 Jmol-3D images Image 1 - FC(F)(F)I
Properties Molecular formula CF3I Molar mass 195.91 g/mol Appearance Colorless odorless gas Density 2.5485 g/cm3 at -78.5 °C
2.3608 g/cm3 at -32.5 °CMelting point -110 °C
Boiling point -22.5 °C
Solubility in water Slightly Vapor pressure 541 kPa Hazards MSDS External MSDS EU Index 602-086-00-0 EU classification Muta. Cat. 3 R-phrases R68 S-phrases (S2), S36/37 Supplementary data page Structure and
propertiesn, εr, etc. Thermodynamic
dataPhase behaviour
Solid, liquid, gasSpectral data UV, IR, NMR, MS  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Trifluoroiodomethane, also referred to as trifluoromethyl iodide is a halomethane with the formula CF3I. It is an experimental alternative to Halon 1301 (CBrF3) in unoccupied areas [1]. It would be used as a gaseous fire suppression flooding agent for in-flight aircraft and electronic equipment fires.
Contents
Chemistry
It is used in the rhodium-catalyzed α-trifluoromethylation of α,β-unsaturated ketones.[1]
In the presence of sunlight or at temperatures above 100°C it undergoes decomposition with hazardous by-products as hydrogen fluoride (HF), hydrogen iodide (HI) and carbonyl fluoride (COF2).
Environmental effects
It contains carbon, fluorine, and iodine atoms. Although iodine is several hundred times more efficient at destroying stratospheric ozone than chlorine, experiments have shown that because the weak C-I bond breaks easily under the influence of water (owing to the electron-attracting fluorine atoms), trifluoroiodomethane has an ozone depleting potential less than one-thousandth that of Halon 1301 (0.008-0.01). Its atmospheric lifetime, at less than 1 month, is less than 1 percent that of Halon 1301, and less even than hydrogen chloride formed from volcanoes.
There is, however, still the problem of the C-F bonds absorbing in the atmospheric window[2]. Thus, even after decomposition, trifluoroiodomethane is likely to be a very effective greenhouse gas.[2]
Further reading
References
- Duan Y. Y., Shi L., Sun L. Q., Zhu M. S., Han L. Z. (March 2000). "Thermodynamic Properties of Trifluoroiodomethane (CF3I)". International Journal of Thermophysics 21 (2): 393–404(12). doi:10.1023/A:1006683529436. http://www.ingentaconnect.com/content/klu/ijot/2000/00000021/00000002/00224129. Retrieved 2007-06-02.
- Duan Y. Y., Shi L., Zhu M. S., Han L. Z. (January 1999). "Surface tension of trifluoroiodomethane (CF3I)". Fluid Phase Equilibria 154 (1): 71–77(7). doi:10.1016/S0378-3812(98)00439-7. http://www.ingentaconnect.com/content/els/03783812/1999/00000154/00000001/art00439;jsessionid=1f3be6692x7fr.alice. Retrieved 2007-06-02.
- Duan Y. Y., Shi L., Sun L. Q., Zhu M. S., Han L. Z. (1997). "Thermal Conductivity of Gaseous Trifluoroiodomethane (CF3I)". J. Chem. Eng. Data 42 (5): 890–893 (4). doi:10.1021/je9700378. http://pubs.acs.org/cgi-bin/abstract.cgi/jceaax/1997/42/i05/abs/je9700378.html. Retrieved 2007-06-02.
- Duan Y. Y., Shi L., Zhu M. S., Han L. Z. (1999). "Critical Parameters and Saturated Density of Trifluoroiodomethane (CF3I)". J. Chem. Eng. Data 44 (3): 501–504. doi:10.1021/je980251b. http://pubs.acs.org/cgi-bin/abstract.cgi/jceaax/1999/44/i03/abs/je980251b.html. Retrieved 2007-06-02.
- Chamber Studies of Photolysis and Hydroxyl Radical Reactions of Trifluoroiodomethane
- ^ 171441 Trifluoroiodomethane 99%
- ^ a b http://webbook.nist.gov/cgi/cbook.cgi?ID=C2314978&Units=SI&Mask=800#Electronic-Spec
External links
Halomethanes Monosubstituted Disubstituted Trisubstituted Categories:- Halomethanes
- Fire suppression agents
- Organofluorides
- Organoiodides
Wikimedia Foundation. 2010.
