- Dibromofluoromethane
-
Dibromofluoromethane 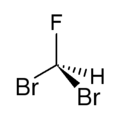 DibromofluoromethaneOther namesFluorodibromomethane
DibromofluoromethaneOther namesFluorodibromomethaneIdentifiers CAS number 1868-53-7 PubChem 61280 ChemSpider 55219 
UNII B0517W9BLP 
Jmol-3D images Image 1
Image 2- C(F)(Br)Br
BrC(Br)F
Properties Molecular formula CHBr2F Molar mass 191.83 g/mol Appearance Liquid Density 2.421 g/cm3 at 20 °C Melting point -78 °C
Boiling point 64.9 °C
Solubility in water Insoluble Supplementary data page Structure and
propertiesn, εr, etc. Thermodynamic
dataPhase behaviour
Solid, liquid, gasSpectral data UV, IR, NMR, MS  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Dibromofluoromethane is a mixed halomethane. It is soluble in alcohol, acetone, benzene and chloroform.
It can be used to prepare bromofluoromethane by reductive debromination with organotin hydride as tributyltin hydride.[1]
Its ozone depletion potential (ODP) is 1.0 and it is included in list of Class I Ozone-Depleting Substances.
References
Halomethanes Monosubstituted Disubstituted Trisubstituted 
This article about an organic halide is a stub. You can help Wikipedia by expanding it. - C(F)(Br)Br
