- Fluoromethane
-
Fluoromethane 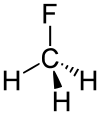
 FluoromethaneOther namesFreon 41
FluoromethaneOther namesFreon 41
Methyl fluoride
Monofluoromethane
Halocarbon 41Identifiers Abbreviations R41 CAS number 593-53-3  , 558-21-4 (2H3)
, 558-21-4 (2H3)  , 20666-44-8 (13C)
, 20666-44-8 (13C) 
PubChem 11638 (2H3)  , 450707 (11C)
, 450707 (11C)  , 12336970 (13C)
, 12336970 (13C)  , 9833918 (17F), 450725 (18F)
, 9833918 (17F), 450725 (18F)  , 11638
, 11638 
ChemSpider 11148 (2H3)  , 396977 (11C)
, 396977 (11C)  , 21170290 (13C)
, 21170290 (13C)  , 8009639 (17F)
, 8009639 (17F)  , 396995 (18F)
, 396995 (18F)  , 11148
, 11148 
EC number 209-796-6 UN number UN 2454 KEGG C11147 
MeSH Fluoromethane ChEBI CHEBI:28826 
ChEMBL CHEMBL116838 
Beilstein Reference 1730725 Gmelin Reference 391 Jmol-3D images Image 1 - FC
Properties Molecular formula CH3F Molar mass 34.03 g/mol Appearance Colorless, odorless gas Density 1.4397 g/L
0.557 g/cm3 (liquid) at saturation pressure at 25 °C
Melting point 131.4 K (-141.8 °C)
Boiling point 195.0 K (-78.2 °C)
Solubility in water 1.66 l/kg (2.295 g/l) Vapor pressure 3.3 MPa Hazards EU classification Very flammable (F+) R-phrases R12 S-phrases S9, S16, S23, S24/25, S26, S28, S33, S36/37/39, S60 NFPA 704  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Fluoromethane, also known as methyl fluoride, Freon 41, Halocarbon-41 and HFC-41, is a non-toxic, liquefiable, and flammable gas at standard temperature and pressure. It is made of carbon, hydrogen, and fluorine. The name stems from the fact that it is methane (CH4) plus fluorine, minus a hydrogen. It is used in the manufacture of semiconductor and electronic products. In the presence of an RF field fluoromethane will dissociate into fluoride ions that selectively etch silicon compound films (reactive-ion etching).
Bond energy of C-F is 552 kJ/mol and its length is 0.139 nm (typically 0.14 nm). Its molecular geometry is tetrahedral.
Its specific heat capacity is Cp = 38.171 J.mol-1.K-1 at 25 °C. Critical point of fluoromethane is at 44.9 °C (318.1 K) and 6.280 MPa.
External links
Halomethanes Monosubstituted Disubstituted Trisubstituted Categories:- Organofluorides
- Halomethanes
- Refrigerants
- Gases
Wikimedia Foundation. 2010.

