- Azo compound
-
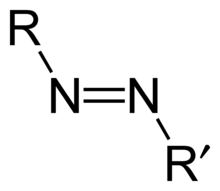
Azo compounds are compounds bearing the functional group R-N=N-R', in which R and R' can be either aryl or alkyl. IUPAC defines azo compounds as: "Derivatives of diazene (diimide), HN=NH, wherein both hydrogens are substituted by hydrocarbyl groups, e.g. PhN=NPh azobenzene or diphenyldiazene."[1] The more stable derivatives contain two aryl groups. The N=N group is called an azo group. The name azo comes from azote, the French name for nitrogen that is derived from the Greek a (not) + zoe (to live).
Contents
As dyes and pigments
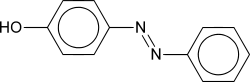
As a consequence of п-delocalization, aryl azo compounds have vivid colors, especially reds, oranges, and yellows. Therefore, they are used as dyes, and are commonly known as azo dyes, an example of which is Disperse Orange 1. Some azo compounds, e.g., methyl orange, are used as acid-base indicators due to the different colors of their acid and salt forms. Most DVD-R/+R and some CD-R discs use blue azo dye as the recording layer. The development of azo dyes was an important step in the development of the chemical industry.
Azo pigments are colorless particles (typically earths or clays), which have been colored using an azo compound. Azo pigments are important in a variety of paints including artist's paints. They have excellent coloring properties, again mainly in the yellow to red range, as well as lightfastness. The lightfastness depends not only on the properties of the organic azo compound, but also on the way they have been adsorbed on the pigment carrier. Many azo pigments are non-toxic, although some, such as dinitroaniline orange, ortho-nitroaniline orange, or pigment orange 1, 2, and 5 have been found to be mutagenic.[2] Likewise, several case studies have linked azo pigments with basal cell carcinoma.[3]
Organic chemistry
Aryl azo compounds
Aryl azo compounds are usually stable, crystalline species. Azobenzene is the prototypical aromatic azo compound. It exists mainly as the trans isomer, but upon photolysis, converts to the cis isomer. Aromatic azo compounds can be synthesized by using an azo coupling reaction, which entails an electrophilic substitution reaction where an aryl diazonium cation attacks another aryl ring, especially those substituted with electron-donating groups.[4] Since diazonium salts are often unstable near room temperature, the azo coupling reactions are typically conducted near ice temperatures. The oxidation of hydrazines (R-NH-NH-R') also gives azo compounds.[5] Azo dyes derived from benzidine are carcinogens; exposure to them has classically been associated with bladder cancer.[6] Accordingly, the production of benzidine azo dyes was discontinued in the 1980s "in the most important western industrialized countries".[7]
Alkyl azo compounds
Aliphatic azo compounds (R and/or R' = aliphatic) are less commonly encountered than the aryl azo compounds. One example is diethyldiazene, EtN=NEt.[8] At elevated temperatures or upon irradiation, the carbon-nitrogen (C-N) bonds in certain alkyl azo compounds cleave with the loss of nitrogen gas to generate radicals. Owing to this process, some aliphatic azo compounds are utilized as radical initiators. Representative is Azobisisobutylonitrile (AIBN) which is widely used as an initiator in polymerization. Because of their instability, especially for aliphatic ones, care should be taken with the handling of azo compounds or an explosion may occur.
See also
References
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2009) "azo compounds".
- ^ Tucson University. "Health & Safety in the Arts, A Searchable Database of Health & Safety Information for Artists". Tucson University Studies. http://www.ci.tucson.az.us/arthazards/paint1.html.
- ^ Eva Engel, Heidi Ulrich, Rudolf Vasold, Burkhard König, Michael Landthaler, Rudolf Süttinger, Wolfgang Bäumler (2008). "Azo Pigments and a Basal Cell Carcinoma at the Thumb". Dermatology 216 (1): 76–80. doi:10.1159/000109363. PMID 18032904.
- ^ H. T. Clarke and W. R. Kirner (1941), "Methyl Red", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=CV1P0374; Coll. Vol. 1: 374
- ^ March, J. “Advanced Organic Chemistry” 5th Ed. J. Wiley and Sons, 1992: New York. ISBN 0-471-60180-2.
- ^ Golka K, Kopps S, Myslak ZW (June 2004). "Carcinogenicity of azo colorants: influence of solubility and bioavailability". Toxicology Letters 151 (1): 203–10. doi:10.1016/j.toxlet.2003.11.016. PMID 15177655. Review.
- ^ Klaus Hunger, Peter Mischke, Wolfgang Rieper, Roderich Raue, Klaus Kunde, Aloys Engel "Azo Dyes” in Ullmann’s Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim.doi:10.1002/14356007.a03_245.
- ^ Ohme, R.; Preuschhof, H.; Heyne, H.-U. (1988), "Azoethane", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=CV6P0078; Coll. Vol. 6: 78
Functional groups Acetyl · Acetoxy · Acryloyl · Acyl · Alcohol · Aldehyde · Alkane · Alkene · Alkyne · Alkoxy group · Amide · Amine · Azo compound · Benzene derivative · Carboxylic acid · Cyanate · Disulfide · Ester · Ether · Epoxide · Haloalkane · Hydrazone · Hydroxyl · Imine · Isocyanate · Isonitrile · Isothiocyanate · Ketone · Methine · Nitrile · Nitro compound · Nitroso compound · Organophosphorus · Oxime · Peroxide · Phosphonous and Phosphonic acid · Pyridine derivative · Sulfone · Sulfonic acid · Sulfoxide · Thiocyanate · Thioester · Thioether · Thiol · Urea
See also Chemical classification Categories:- Azo compounds
- Azo dyes
- Pigments
- Organic pigments
Wikimedia Foundation. 2010.