- Photochemistry
-
Photochemistry, a sub-discipline of chemistry, is the study of chemical reactions that proceed with the absorption of light by atoms or molecules.[1]. Everyday examples include photosynthesis, the degradation of plastics and the formation of vitamin D with sunlight.
Contents
Principles
Light is a type of electromagnetic radiation, a source of energy. The Grotthuss–Draper law (for chemists Theodor Grotthuss and John W. Draper), states that light must be absorbed by a chemical substance in order for a photochemical reaction to take place. For each photon of light absorbed by a chemical system, no more than one molecule is activated for a photochemical reaction, as defined by the quantum yield.
Chemical reactions occur only when a molecule is provided the necessary "activation energy". A simple example can be the combustion of gasoline (a hydrocarbon) into carbon dioxide and water. In this reaction, the activation energy is provided in the form of heat or a spark. In case of photochemical reactions light provides the activation energy. Simplistically, light is one mechanism for providing the activation energy required for many reactions. If laser light is employed, it is possible to selectively excite a molecule so as to produce a desired electronic and vibrational state. Equally, the emission from a particular state may be selectively monitored, providing a measure of the population of that state. If the chemical system is at low pressure, this enables scientists to observe the energy distribution of the products of a chemical reaction before the differences in energy have been smeared out and averaged by repeated collisions.
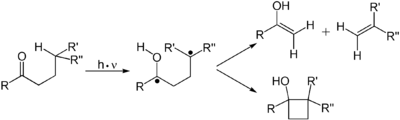
The absorption of a photon of light by a reactant molecule may also permit a reaction to occur not just by bringing the molecule to the necessary activation energy, but also by changing the symmetry of the molecule's electronic configuration, enabling an otherwise inaccessible reaction path, as described by the Woodward-Hoffmann selection rules. A 2+2 cycloaddition reaction is one example of a pericyclic reaction that can be analyzed using these rules or by the related frontier molecular orbital theory.
Photochemical reactions involve electronic reorganization initiated by electromagnetic radiation. The reactions are several orders of magnitude faster than thermal reactions; reactions as fast as 10−9 seconds and associated processes as fast as 10−15 seconds are often observed.
Spectral regions
Photochemists typically work in only a few sections of the electromagnetic spectrum. Some of the most widely used sections, and their wavelengths, are the following:
- Ultraviolet: 100–400 nm
- Visible Light: 400–700 nm
- Near infrared: 700–2500 nm
Applications
Many important processes involve photochemistry. The premier example is photosynthesis, in which most plants use solar energy to convert carbon dioxide and water into glucose, disposing of oxygen as a side-product. Humans rely on photochemistry for the formation of vitamin D. In fireflies, an enzyme in the abdomen catalyzes a reaction that results in bioluminescence.[2]
Photochemistry can also be highly destructive. Medicine bottles are often made with darkened glass to prevent the drugs from photodegradation. A pervasive reaction is the generation of singlet oxygen by photosensitized reactions of triplet oxygen. Typical photosensitizers include tetraphenylporphyrin and methylene blue. The resulting singlet oxygen is an aggressive oxidant, capable of converting C-H bonds into C-OH groups.In photodynamic therapy, light is used to destroy tumors by the action of singlet oxygen.
Many polymerizations are started by photoinitiatiors, which decompose upon absorbing light to produce the free radicals for Radical polymerization.
In the area of photochemistry, a photochemical reaction is a chemical reaction that is induced by light. Photochemical reactions are valuable in organic and inorganic chemistry because they proceed differently than thermal reactions. Photochemical reactions are not only very useful but also can be a serious nuisance, as in the photodegradation of many materials, e.g. polyvinyl chloride. A large-scale application of photochemistry is photoresist technology, used in the production of microelectronic components. Vision is initiated by a photochemical reaction of rhodopsin.[3]
Experimental set-up
Photochemical reactions require a light source that emits wavelengths corresponding to an electronic transition in the reactant. In the early experiments (and in everyday life), sunlight was the light source, although it is polychromatic. Mercury-vapor lamps are more common in the laboratory. Low pressure mercury vapor lamps mainly emit at 254 nm. For polychromatic sources, wavelength ranges can be selected using filters. Alternatively, LEDs and Rayonet lamps emit monochromatically.
The emitted light must of course reach the targeted functional group without being blocked by the reactor, medium, or other functional groups present. For many applications, quartz is used for the reactors as well as to contain the lamp. Pyrex absorbs at wavelengths shorter than 275 nm. The solvent is an important experimental parameter. Solvents are potential reactants and for this reason, chlorinated solvents are avoided because the C-Cl bond can lead to chlorination of the substrate. Strongly absorbing solvents prevent photons from reaching the substrate. Hydrocarbon solvents absorb only at short wavelengths and are thus preferred for photochemical experiments requiring high energy photons. Solvents containing unsaturation absorb at longer wavelengths and can usefully filter out short wavelengths. For example, cyclohexane and acetone "cut off" (absorb strongly) at wavelengths shorter than 215 and 330 nm, respectively.
Excitation
Photoexcitation is the first step in a photochemical process where the reactant is elevated to a state of higher energy, an excited state. The photon can be absorbed directly by the reactant or by a photosensitizers, which absorbs the photon and transfers the energy to the reactant. The opposite process is called quenching when a photoexited state is deactivated by a chemical reagent.
Most photochemical transformations occur through a series of simple steps known as primary photochemical processes. One common example of these processes is the excited state proton transfer (ESPT).
Organic photochemistry
Main article: Mechanistic organic photochemistryExamples of photochemical organic reactions are electrocyclic reactions, photoisomerization and Norrish reactions.
Alkenes undergo many important reactions that proceed via a photon-induced π to π* transition. The first electronic excited state of an alkene lack the π-bond, so that rotation about the C-C bond is rapid and the molecule engages in reactions not observed thermally. These reactions include cis-trans isomerization, cycloaddition to other (ground state) alkene to give cyclobutane derivatives. The cis-trans isomerization of a (poly)alkene is involved in retinal, a component of the machinery of vision. The dimerization of alkenes is relevant to the photodamage of DNA, where thymine dimers are observed upon illuminating DNA to UV radiation. Such dimers interfere with transcription. The beneficial effects of sunlight are associated with the photochemically induced retro-cyclization (decyclization) reaction of ergosterol to give vitamin D. In the DeMayo reaction, an alkene reacts with a 1,3-diketone reacts via its enol to yield a 1,5-diketone. Still another common photochemical reaction is Zimmerman's Di-pi-methane rearrangement.
In an industrial application, about 100,000 tonnes of benzyl chloride are prepared annually by the gas-phase photochemical reaction of toluene with chlorine.[4] The light is absorbed by chlorine molecule, the low energy of this transition being indicted by the yellowish color of the gas. The photon induces homolysis of the Cl-Cl bond, and the resulting chlorine radical converts toluene to the benzyl radical:
- Cl2 + hν → 2 Cl·
- C6H5CH3 + Cl· → C6H5CH2· + HCl
- C6H5CH2· + Cl· → C6H5CH2Cl
Mercaptans can be produced by photochemical addition of hydrogen sulfide (H2S) to alpha olefins.
Inorganic and organometallic photochemistry
Coordination complexes and organometallic compounds are also photoreactive. These reactions can entail cis-trans isomerization. More commonly photoreactions result in dissociation of ligands, since the photon excites an electron on the metal to an orbital that is antibonding with respect to the ligands. Thus, metal carbonyls that resist thermal substitution undergo decarbonylation upon irradiation with UV light. UV-irradiation of a THF solution of molybdenum hexacarbonyl gives the THF complex, which is synthetically useful:
- Mo(CO)6 + THF → Mo(CO)5(THF) + CO
In a related reaction, photolysis of iron pentacarbonyl affords diiron nonacarbonyl (see figure):
- 2 Fe(CO)5 → Fe2(CO)9 + CO
Carbon Nanotubes
Certain species of Carbon Nanotubes also undergo photochemical reactions when exposed to intense pulsed light from a laser, arc lamp, or flashbulb[5]. In the presence of air, Carbon Nanotubes will undergo combustion at high temperature (700-1500C). Under inert atmospheres, the nanotubes will break open and reorganize into larger "nanohorn" structures.
History
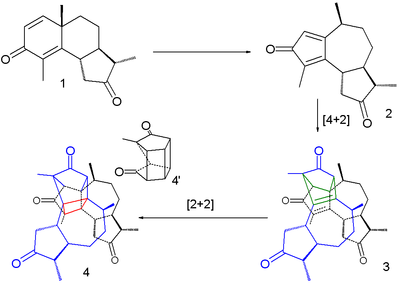
Although bleaching has long been practiced, the first photochemical reaction was described by Trommsdorf in 1834.[6] He observed that crystals of the compound α-santonin when exposed to sunlight turned yellow and burst. In a 2007 study the reaction was described as a succession of three steps taking place within a single crystal.[7]
The first step is a rearrangement reaction to a cyclopentadienone intermediate 2, the second one a dimerization in a Diels-Alder reaction (3) and the third one a intramolecular [2+2]cycloaddition (4). The bursting effect is attributed to a large change in crystal volume on dimerization.
References
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "photochemistry".
- ^ David Stanley Saunders Insect clocks, Elsevier, 2002, ISBN 0444504079 p. 179
- ^ Christophe Dugave Cis-trans isomerization in biochemistry, Wiley-VCH, 2006 ISBN 3527313044 p. 56
- ^ M. Rossberg et al. “Chlorinated Hydrocarbons” in Ullmann’s Encyclopedia of Industrial Chemistry 2006, Wiley-VCH, Weinheim. doi:10.1002/14356007.a06_233.pub2
- ^ Nanotubes in a Flash-Ignition and Reconstruction Ajayan, et al. Science 269 (705) 26 Apr 2002 http://www.owlnet.rice.edu/~rv4/Ajayan/flash.pdf
- ^ Trommsdorf, Ann. Chem. Pharm. 1834, 11
- ^ The Photoarrangement of -Santonin is a Single-Crystal-to-Single-Crystal Reaction: A Long Kept Secret in Solid-State Organic Chemistry Revealed Arunkumar Natarajan, C. K. Tsai, Saeed I. Khan, Patrick McCarren, K. N. Houk, and Miguel A. Garcia-Garibay J. Am. Chem. Soc., 129 (32), 9846 -9847, 2007. doi:10.1021/ja073189o
See also
- Journal of Photochemistry and Photobiology
- Photoelectrochemical cell
- Photochemical and Photobiological Sciences
- Trends in Photochemistry & Photobiology
- Photochemistry and Photobiology
- Photochemical Logic Gates
- Photosynthesis
Branches of Chemistry Physical chemistry Chemical kinetics · Chemical physics · Electrochemistry · Materials science · Photochemistry · Quantum chemistry · Solid-state chemistry · Spectroscopy · Surface chemistry · Thermochemistry
Organic chemistry Biochemistry · Biophysical chemistry · Bioinorganic chemistry · Bioorganic chemistry · Chemical biology · Medicinal chemistry · Organic chemistry · Organometallic chemistry · Pharmacy · Physical organic chemistry · Polymer chemistry ·
Inorganic chemistry Others Categories:
Wikimedia Foundation. 2010.