- Rearrangement reaction
-
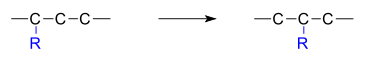
A rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule.[1] Often a substituent moves from one atom to another atom in the same molecule. In the example below the substituent R moves from carbon atom 1 to carbon atom 2:
Intermolecular rearrangements also take place.
A rearrangement is not well represented by simple and discrete electron transfers (represented by curly arrows in organic chemistry texts). The actual mechanism of alkyl groups moving, as in Wagner-Meerwein rearrangement, probably involves transfer of the moving alkyl group fluidly along a bond, not ionic bond-breaking and forming. In pericyclic reactions, explanation by orbital interactions give a better picture than simple discrete electron transfers. It is, nevertheless, possible to draw the curved arrows for a sequence of discrete electron transfers that give the same result as a rearrangement reaction, although these are not necessarily realistic. In allylic rearrangement, the reaction is indeed ionic.
Three key rearrangement reactions are 1,2-rearrangements, pericyclic reactions and olefin metathesis.
Contents
1,2-rearrangements
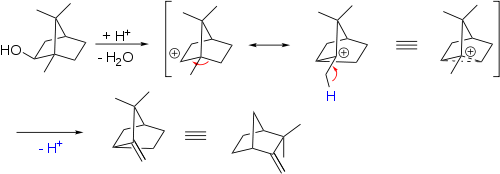
Main article: 1,2-rearrangementA 1,2-rearrangement is an organic reaction where a substituent moves from one atom to another atom in a chemical compound. In a 1,2 shift the movement involves two adjacent atoms but moves over larger distances are possible. Examples are the Wagner-Meerwein rearrangement:
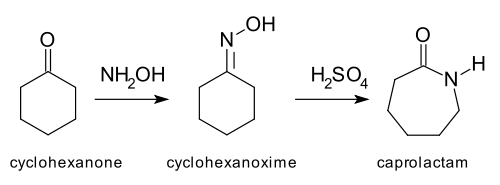
and the Beckmann rearrangement:
Pericyclic reactions
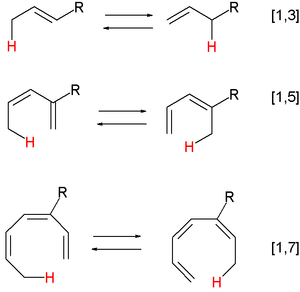
Main article: pericyclic reactionsA pericyclic reaction is a type of reaction with multiple carbon-carbon bond making and breaking wherein the transition state of the molecule has a cyclic geometry, and the reaction progresses in a concerted fashion. Examples are hydride shifts
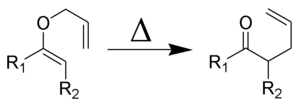
and the Claisen rearrangement:
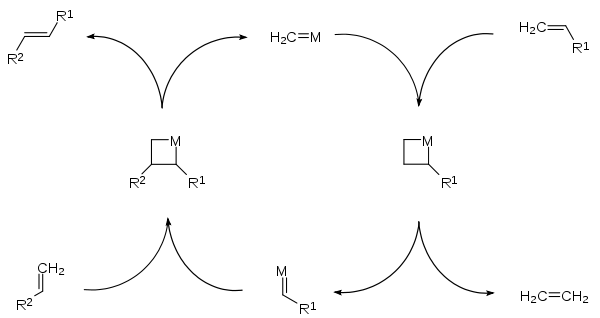
Olefin metathesis
Main article: Olefin metathesisOlefin metathesis is a formal exchange of the alkylidene fragments in two alkenes. It is a catalytic reaction with carbene, or more accurately, transition metal carbene complex intermediates.
See also
- Beckmann rearrangement
- Curtius rearrangement
- Hofmann rearrangement
- Lossen rearrangement
- Schmidt reaction
- Tiemann rearrangement
- Wolff rearrangement
- Photochemical rearrangements
References
- ^ March, Jerry (1985), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (3rd ed.), New York: Wiley, ISBN 0-471-85472-7
Topics in Organic Reactions Addition reaction - Elimination reaction - Polymerization - Reagents - Rearrangement reaction - Redox reaction - Regioselectivity - Stereoselectivity - Stereospecificity - Substitution reaction - List of organic reactions
Categories:- Rearrangement reactions
Wikimedia Foundation. 2010.