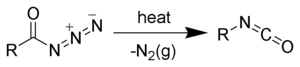- Curtius rearrangement
-
The Curtius rearrangement (or Curtius reaction or Curtius degradation), as first defined by Theodor Curtius, is a chemical reaction that involves the rearrangement of an acyl azide to an isocyanate.[1][2] Several reviews have been published.[3][4]
The isocyanate can be trapped by a variety of nucleophiles. Water is often added in order to hydrolyze the isocyanate to an amine.[5] When done in the presence of tert-butanol, the reaction generates Boc-protected amines, useful intermediates in organic synthesis.[6][7]
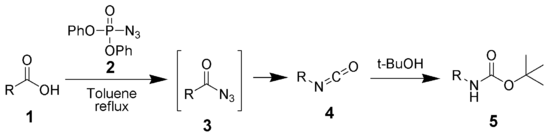
Carboxylic acids 1 can be easily converted to acyl azides 3 using diphenylphosphoryl azide 2.[8][9][10]
Likewise, when the Curtius reaction is performed in the presence of benzyl alcohol, Cbz-protected amines are formed.[11]
Contents
Reaction mechanism
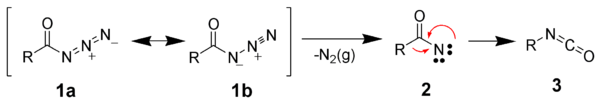
The Curtius rearrangement may be thought of as a two-step process, the first step being the loss of nitrogen gas, forming an acyl nitrene (2), and the second step being the rearrangement of acyl nitrenes by migration of R-group to form the desired isocyanate (3). However, current evidence indicates that these two steps are likely concerted (i.e., they occur at the same time), and no free nitrene intermediate is formed.[12]
Scope
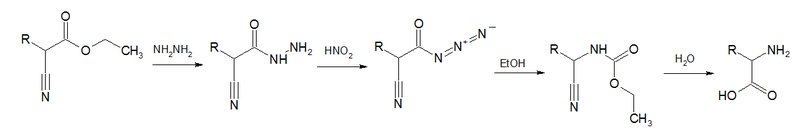
In one variation called the Darapsky degradation (A. Darapsky, 1936), a Curtius rearrangement takes place as one of the steps from an α-cyanoester to an amino acid.[13]
References
- ^ Curtius, T. (1890). Ber. 23: 3023.
- ^ Curtius, T. (1894). "20. Hydrazide und Azide organischer S�uren I. Abhandlung". Journal f�r Praktische Chemie 50: 275–294. doi:10.1002/prac.18940500125.
- ^ Smith, P. A. S. (1946). Org. React. 3: 337–449.
- ^ Scriven, E. R. I. C. F. V.; Turnbull, K. E. N. N. E. T. H. (1988). "Azides: their preparation and synthetic uses". Chemical Reviews 88 (2): 297. doi:10.1021/cr00084a001.
- ^ Kaiser, C.; Weinstock, J. (1988), "Amines from mixed carboxylic-carbonic anhydrides: 1-phenylcyclopentylamine", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv6p0910; Coll. Vol. 6: 910
- ^ Am Ende, D. A. V. I. D. J.; Devries, K. E. I. T. H. M.; Clifford, P. A. M. E. L. A. J.; Brenek, S. T. E. V. E. N. J. (1998). "A Calorimetric Investigation to Safely Scale-Up a Curtius Rearrangement of Acryloyl Azide". Organic Process Research & Development 2 (6): 382. doi:10.1021/op970115w.
- ^ Lebel, H.; Leogane, O. (2005). "Boc-protected amines via a mild and efficient one-pot Curtius rearrangement". Organic letters 7 (19): 4107–4110. doi:10.1021/ol051428b. PMID 16146363.
- ^ Shioiri, T.; Yamada, S. (1990), "Diphenyl phosphorazidate", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv7p0206; Coll. Vol. 7: 206
- ^ Shioiri, T.; Ninomiya, K.; Yamada, S. (1972). "Diphenylphosphoryl azide. New convenient reagent for a modified Curtius reaction and for peptide synthesis". Journal of the American Chemical Society 94 (17): 6203. doi:10.1021/ja00772a052. PMID 5054412.
- ^ Ninomiya, K. (1974). "Phosphorus in organic synthesis—VII , Diphenyl phosphorazidate (DPPA). A new convenient reagent for a modified curtius reaction". Tetrahedron 30 (14): 2151–2157. doi:10.1016/S0040-4020(01)97352-1.
- ^ Jessup, P. J.; Petty, C. B.; Roos, J.; Overman, L. E. (1988), "1-N-Acylamino-1,3-dienes from 2,4-pentadienoic acids by the Curtius rearrangement: benzyl trans-1,3-butadiene-1-carbamate", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv6p0095; Coll. Vol. 6: 95
- ^ Smith, Michael B.; March, Jerry (2007). March's Advanced Organic Chemistry (6th ed.). Hoboken, New Jersey: Wiley. p. 1609. ISBN 9780471720911.
- ^ http://www.chempensoftware.com/reactions/RXN051.htm
See also
- Beckmann rearrangement
- Hofmann rearrangement
- Lossen rearrangement
- Schmidt reaction
- Tiemann rearrangement
- Wolff rearrangement
External links
Categories:- Rearrangement reactions
- Name reactions
Wikimedia Foundation. 2010.