- Substitution reaction
-
In a substitution reaction, a functional group in a particular chemical compound is replaced by another group.[1][2] In organic chemistry, the electrophilic and nucleophilic substitution reactions are of prime importance. Organic substitution reactions are classified in several main organic reaction types depending on whether the reagent that brings about the substitution is considered an electrophile or a nucleophile, whether a reactive intermediate involved in the reaction is a carbocation, a carbanion or a free radical or whether the substrate is aliphatic or aromatic. Detailed understanding of a reaction type helps to predict the product outcome in a reaction. It also is helpful for optimizing a reaction with regard to variables such as temperature and choice of solvent.
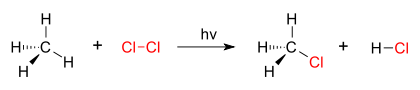
A good example of a substitution reaction is the photochemical chlorination of methane forming methyl chloride.
chlorination of methane by chlorine Contents
Nucleophilic substitution
Nucleophilic substitution happens when the reagent is a nucleophile, which means, an atom or molecule with free electrons.
A nucleophile reacts with an aliphatic substrate in a nucleophilic aliphatic substitution reaction. These substitutions can be produced by two different mechanisms: unimolecular nucleophilic substitution (SN1) and bimolecular nucleophilic substitution (SN2). The SN1 mechanism has two steps. In the first step, the leaving group departs, forming a carbocation. In the second step, the nucleophilic reagent attacks the carbocation and forms a sigma bond. This mechanism can result in either inversion or retention of configuration. An SN2 reaction has just one step. The attack of the reagent and the expulsion of the leaving group happen simultaneously. This mechanism always results in inversion of configuration.
When the substrate is an aromatic compound the reaction type is nucleophilic aromatic substitution. Carboxylic acid derivatives react with nucleophiles in nucleophilic acyl substitution. This kind of reaction can be useful in preparing compouds
Electrophilic substitution
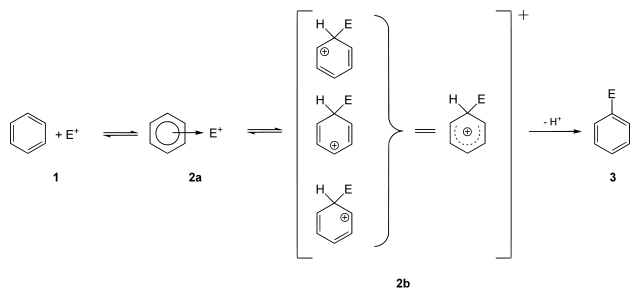
Electrophiles are involved in electrophilic substitution reactions and particularly in electrophilic aromatic substitutions.
Electrophilic aromatic substitution Electrophilic reactions to other unsaturated compounds than arenes generally lead to electrophilic addition rather than substitution.
Radical substitution
A radical substitution reaction involves radicals. An example is the Hunsdiecker reaction.
Organometallic substitution
Coupling reactions are a class of metal-catalyzed reactions involving an organometallic compound RM and an organic halide R'X that together react to a compound of the type R-R' with formation of a new carbon-carbon bond. Examples are the Heck reaction and the Ullmann reaction. Many variations exist.[3]
Substituted compounds
Substituted compounds are chemical compounds where one or more hydrogen atoms of a core structure have been replaced with a functional group like alkyl, hydroxy, or halogen.
For example benzene is a simple aromatic ring and substituted benzenes are a heterogeneous group of chemicals with a wide spectrum of uses and properties:
compound general formula general structure Benzene C6H6 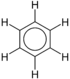
Toluene C6H5-CH3 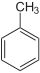
o-Xylene C6H4(-CH3)2 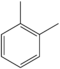
Mesitylene C6H3(-CH3)3 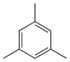
Phenol C6H5-OH 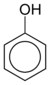
Just a few substituted benzene compounds References
- ^ March, Jerry (1985), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (3rd ed.), New York: Wiley, ISBN 0-471-85472-7
- ^ Imyanitov, Naum S. (1993). "Is This Reaction a Substitution, Oxidation-Reduction, or Transfer?". J. Chem. Educ. 70 (1): 14–16. doi:10.1021/ed070p14.
- ^ Elschenbroich, C.; Salzer, A. (1992). Organometallics: A Concise Introduction (2nd ed.). Weinheim: Wiley-VCH. ISBN 3-527-28165-7.
Basic reaction mechanisms Nucleophilic substitution Elimination reaction Related topics Chemical kinetics Topics in Organic Reactions Addition reaction - Elimination reaction - Polymerization - Reagents - Rearrangement reaction - Redox reaction - Regioselectivity - Stereoselectivity - Stereospecificity - Substitution reaction - List of organic reactions
Categories:- Chemical reactions
- Substitution reactions
Wikimedia Foundation. 2010.