- Mesitylene
-
Mesitylene 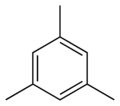
 1,3,5-TrimethylbenzeneOther namesMesitylene
1,3,5-TrimethylbenzeneOther namesMesitylene
sym-TrimethylbenzeneIdentifiers CAS number 108-67-8 
PubChem 7947 ChemSpider 7659 
EC number 203-604-4 KEGG C14508 
ChEBI CHEBI:34833 
Jmol-3D images Image 1 - Cc1cc(cc(c1)C)C
Properties Molecular formula C9H12 Molar mass 120.19 g/mol Density 0.8637 g/cm³ at 20 °C Melting point -44.8 °C
Boiling point 164.7 °C
Hazards MSDS Safety data from Oxford University  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Mesitylene or 1,3,5-trimethylbenzene (C9H12) is an aromatic hydrocarbon with three methyl substituents attached to the benzene ring. It is prepared by distillation of acetone with sulfuric acid or by trimerization of propyne in sulfuric acid, which, in both cases, acts as a catalyst and dehydrating agent. It is commonly used as a solvent in research and industry. It is flammable and an irritant; it is a low-freezing liquid.
In the electronics industry, mesitylene has also been used as a developer for photopatternable silicones due to its solvent properties.
1,3,5-Trimethylbenzene is also a major urban volatile organic compound (VOC) which results from combustion. It plays a significant role in aerosol and tropospheric ozone formation as well as other reactions in atmospheric chemistry.
The mesityl group (Mes) is a functional group found as an attachment in many organic compounds.
The three aromatic hydrogen atoms of mesitylene are in identical chemical shift environments. Therefore, they only give a single peak near 6.8 ppm in the 1H NMR spectrum. For this reason, mesitylene is sometimes used as an internal standard in NMR samples that contain aromatic protons.[1]
References
Categories:- Hydrocarbon solvents
- Pollutants
- Smog
- Alkylbenzenes
Wikimedia Foundation. 2010.
